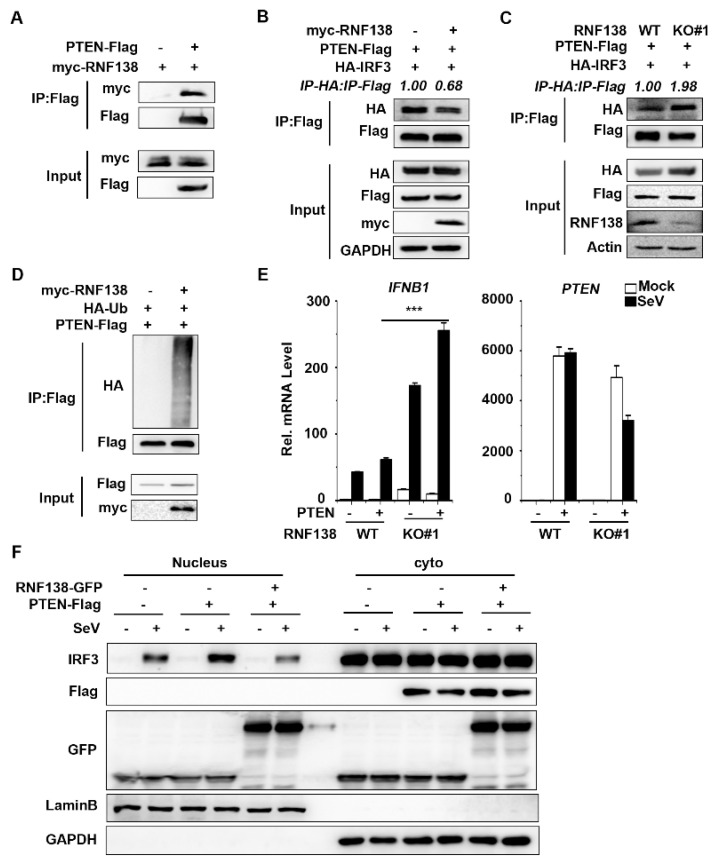
Figure 5.
RNF138 inhibits IRF3 activation by ubiquitinating PTEN. (A) Co-immunoprecipitation analysis of the interaction between RNF138 and PTEN in HEK293T cells transfected with myc-RNF138 and PTEN-Flag or a control vector for 24 h. (B) RNF138 inhibited the association of PTEN with IRF3. HEK293T cells were transfected with PTEN-Flag and HA-IRF3 with a control and myc-RNF138 plasmid for 24 h before immunoblotting and co-immunoprecipitation analysis with the indicated antibodies. (C) RNF138 deficiency promotes the association of PTEN with IRF3. RNF138-KO and control HEK293T cells were transfected with PTEN-Flag and HA-IRF3 for 24 h before immunoblotting and co-immunoprecipitation analysis with the indicated antibodies. (D) The effects of RNF138 on the polyubiquitination of PTEN. HEK293T cells were transfected with PTEN-Flag and HA-Ub together with control or myc-RNF138 plasmid for 24 h, followed by immunoblotting and co-immunoprecipitation analysis with the indicated antibodies. (E) The effects of RNF138 deficiency on the PTEN-mediated transcription of IFNB1 gene. RNF138-KO and control HEK293T cells were transfected with PTEN-Flag for 24 h and then infected with SeV for 8 h before qPCR analysis. (F) The effects of RNF138 on the PTEN-mediated nuclear translocation of IRF3. HEK293T cells were transfected with the control or PTEN-Flag plasmid together with GFP or RNF138-GFP plasmid for 24 h then infected with SeV for 8 h. Immunoblot analysis of IRF3 in cytoplasmic (Cyto) and nucleus fractions with the indicated antibodies. *** p < 0.001 (unpaired t-test). Data represent at least two experiments with similar results (mean ± SD, n = 3 independent samples in (A,D,E)).