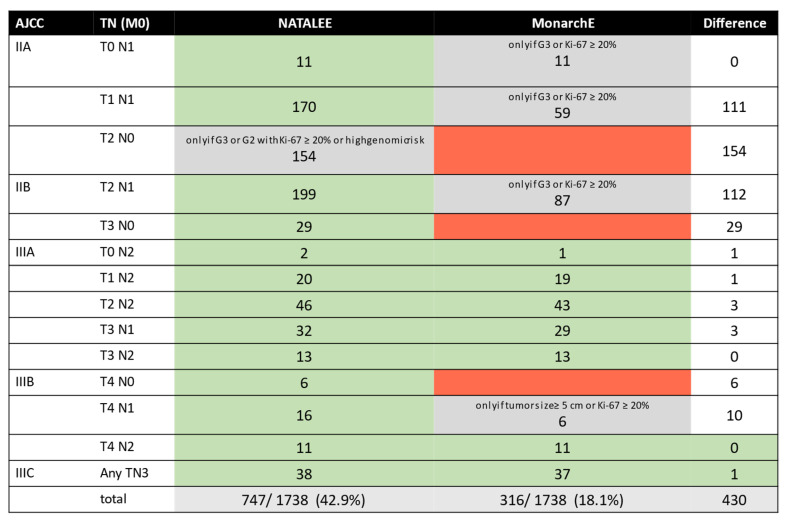
Figure 2.
Comparison of patients potentially eligible for Ribociclib versus Abemaciclib therapy analogous with the NATALEE and MonarchE trials: The absolute numbers of potentially eligible patients, based on the inclusion criteria of the NATALEE study versus the MonarchE study, are depicted. According to the study protocols, the NATALEE study primarily considered the post-operative, pathological tumor stage as an inclusion criterion. In the case of neoadjuvant chemotherapy, the clinical TNM stage was also accepted. For Abemaciclib, only the post-operative, pathological TNM stage was considered, in line with the MonarchE study protocol. Fields shaded in gray indicate that the inclusion criteria are met only under specific conditions, as detailed in the table, while fields highlighted in red indicate that the inclusion criteria are not met for that specific tumor stage.