Abstract
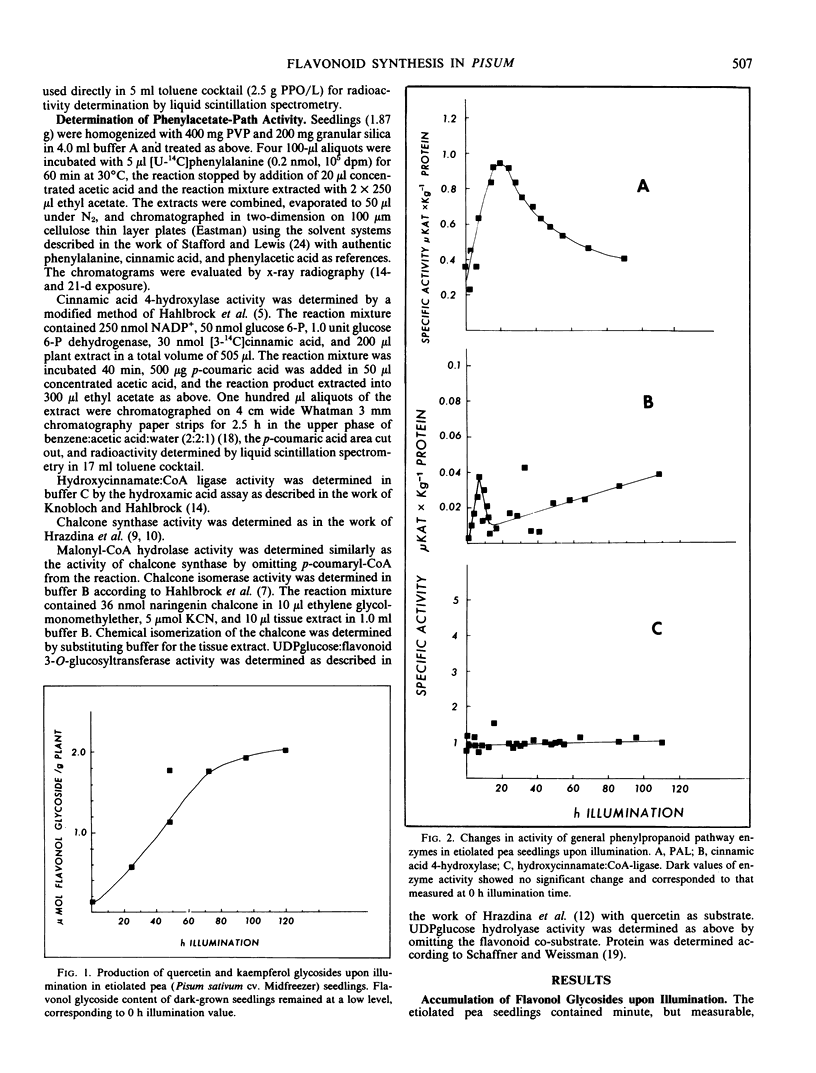
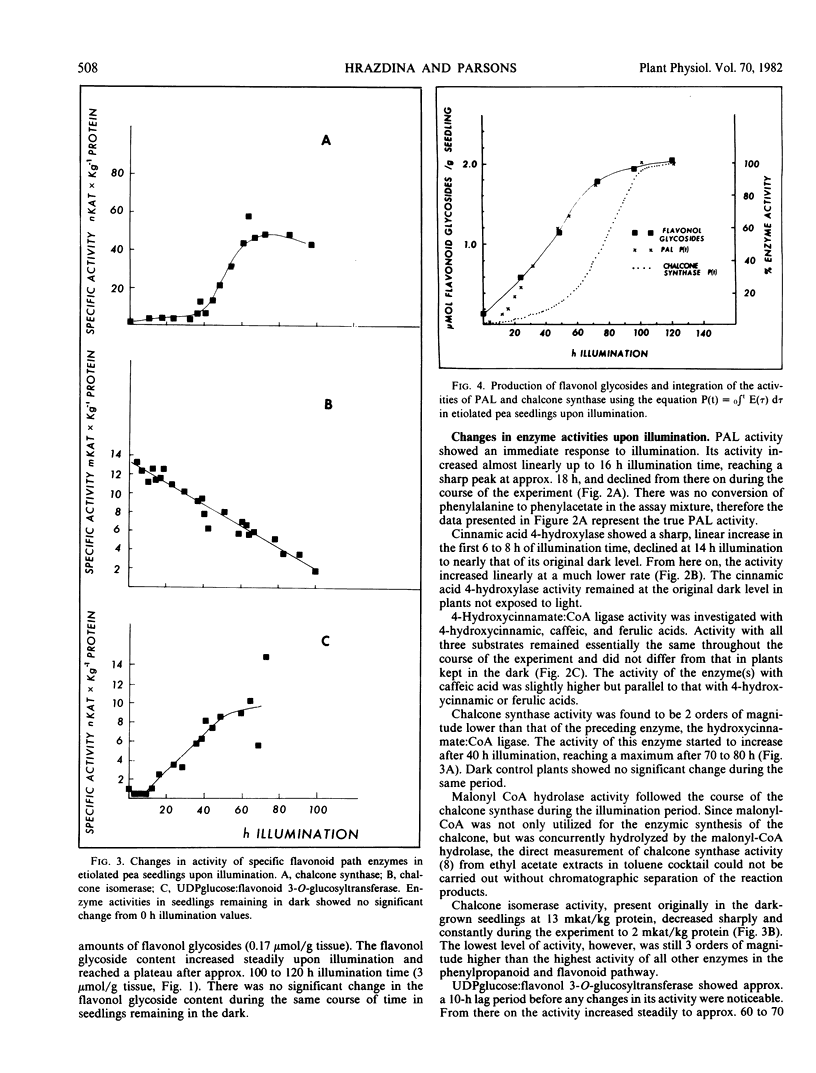
Etiolated pea (Pisum sativum cv. Midfreezer) seedlings respond to illumination with white light by changes in the activity of phenylpropanoid and flavonoid synthesizing enzymes. Unlike in cell cultures, changes in enzyme activity in pea seedlings are not concerted. Phenylalanine ammonia-lyase (EC 4.3.1.5) activity peaked approximately 18 hours after onset of illumination. The phenylacetate path did not interfere with the measurement of phenylalanine ammonia-lyase activity. Activity of cinnamic acid 4-hydroxylase (EC 1.14.13.11) showed an early peak after 8 hours illumination, declined thereafter sharply, then gradually increased during the remainder of the experiment. Activities of chalcone synthase and UDP glucose:flavonol 3-O-glucosyltransferase (EC 2.4.1.91) increased steadily and reached a plateau after approximately 70 hours illumination time. Activity of 4-hydroxycinnamate:coenzyme A ligase (EC 6.2.1.12) remained relatively unchanged, whereas that of chalcone isomerase (EC 5.5.1.6) declined steadily during the course of the experiment. The relative in vitro enzyme activities suggest that the rate-limiting step for the phenylpropanoid path is the cinnamic acid 4-hydroxylase, that of the flavonoid pathway is the chalcone synthase. Integration of enzyme activity curves, however, show that only the curve deriving from phenylanine ammonia-lyase activity matches closely the production of the flavonol glycosides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hahlbrock K., Ebel J., Ortmann R., Sutter A., Wellmann E., Grisebach H. Regulation of enzyme activities related to the biosynthesis of flavone glycosides in cell suspension cultures of parsley (Petroselinum hortense). Biochim Biophys Acta. 1971 Jul 20;244(1):7–15. doi: 10.1016/0304-4165(71)90114-0. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Knobloch K. H., Kreuzaler F., Potts J. R., Wellmann E. Coordinated induction and subsequent activity changes of two groups of metabolically interrelated enzymes. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur J Biochem. 1976 Jan 2;61(1):199–206. doi: 10.1111/j.1432-1033.1976.tb10012.x. [DOI] [PubMed] [Google Scholar]
- Heller W., Hahlbrock K. Highly purified "flavanone synthase" from parsley catalyzes the formation of naringenin chalcone. Arch Biochem Biophys. 1980 Apr 1;200(2):617–619. doi: 10.1016/0003-9861(80)90395-1. [DOI] [PubMed] [Google Scholar]
- Hrazdina G., Kreuzaler F., Hahlbrock K., Grisebach H. Substrate specificity of flavanone synthase from cell suspension cultures of parsley and structure of release products in vitro. Arch Biochem Biophys. 1976 Aug;175(2):392–399. doi: 10.1016/0003-9861(76)90526-9. [DOI] [PubMed] [Google Scholar]
- Knobloch K. H., Hahlbrock K. Isoenzymes of p-coumarate: CoA ligase from cell suspension cultures of Glycine max. Eur J Biochem. 1975 Mar 17;52(2):311–320. doi: 10.1111/j.1432-1033.1975.tb03999.x. [DOI] [PubMed] [Google Scholar]
- Potts J. R., Weklych R., Conn E. E., Rowell J. The 4-hydroxylation of cinnamic acid by sorghum microsomes and the requirement for cytochrome P-450. J Biol Chem. 1974 Aug 25;249(16):5019–5026. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Stafford H. A., Lewis L. L. Interference by a phenylacetate pathway in isotopic assays for phenylalanine ammonia-lyase in leaf extracts. Plant Physiol. 1977 Dec;60(6):830–834. doi: 10.1104/pp.60.6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]


