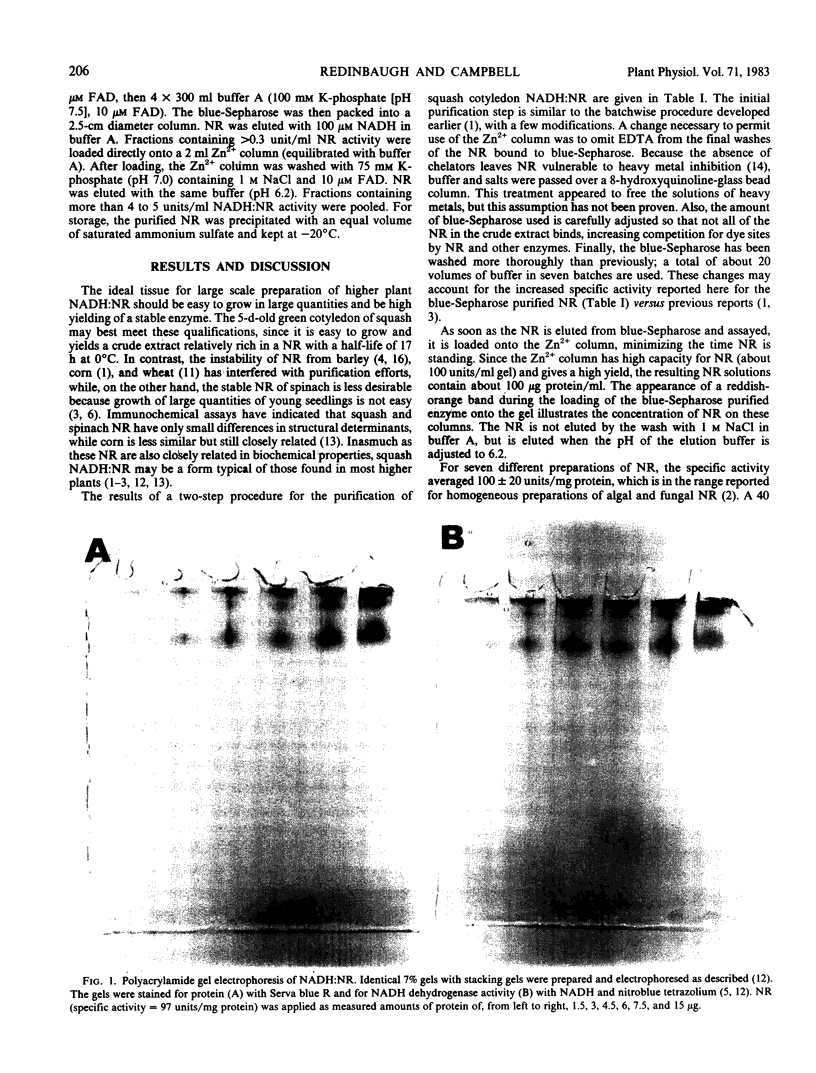
Abstract
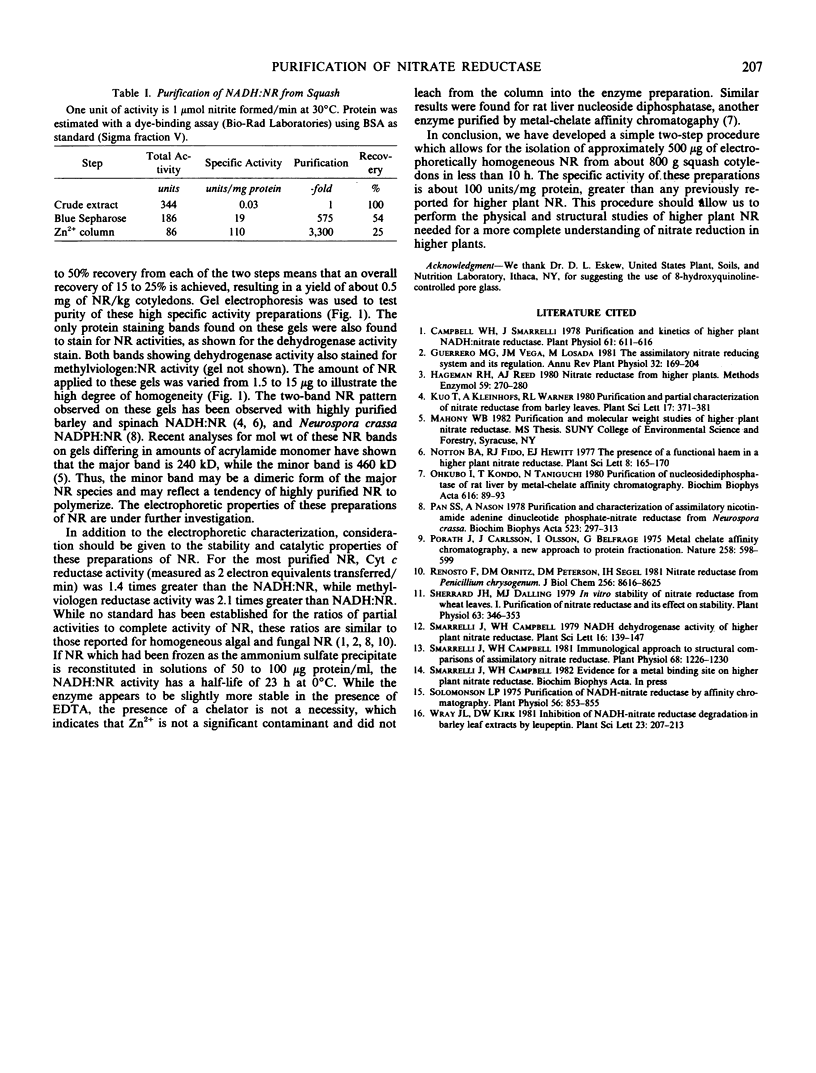
NADH:nitrate reductase (EC 1.6.6.1) was isolated and purified from the green cotyledons of 5-day-old squash seedlings (Cucurbita maxima L.). The 10-hour purification procedure consisted of two steps: direct application of crude enzyme to blue Sepharose and specific elution with NADH followed by direct application of this effluent to a Zn2+ column with elution by decreasing the pH of the phosphate buffer from 7.0 to 6.2. The high specific activity (100 micromoles per minute per milligram protein) and high recovery (15-25%) of electrophoretically homogeneous nitrate reductase show that the enzyme was not damaged by exposure to the bound zinc. With this procedure, homogeneous nitrate reductase can be obtained in yields of 0.5 milligram per kilogram cotyledons.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell W. H., Smarrelli J. Purification and Kinetics of Higher Plant NADH:Nitrate Reductase. Plant Physiol. 1978 Apr;61(4):611–616. doi: 10.1104/pp.61.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo I., Kondo T., Taniguchi N. Purification of nucleosidediphosphatase of rat liver by metal-chelate affinity chromatography. Biochim Biophys Acta. 1980 Nov 6;616(1):89–93. doi: 10.1016/0005-2744(80)90266-1. [DOI] [PubMed] [Google Scholar]
- Pan S. S., Nason A. Purification and characterization of homogeneous assimilatory reduced nicotinamide adenine dinucleotide phosphate-nitrate reductase from Neurospora crassa. Biochim Biophys Acta. 1978 Apr 12;523(2):297–313. doi: 10.1016/0005-2744(78)90033-5. [DOI] [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Renosto F., Ornitz D. M., Peterson D., Segel I. H. Nitrate reductase from Penicillium chrysogenum. Purification and kinetic mechanism. J Biol Chem. 1981 Aug 25;256(16):8616–8625. [PubMed] [Google Scholar]
- Sherrard J. H., Dalling M. J. In vitro stability of nitrate reductase from wheat leaves: I. Stability of highly purified enzyme and its component activities. Plant Physiol. 1979 Feb;63(2):346–353. doi: 10.1104/pp.63.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarrelli J., Campbell W. H. Immunological approach to structural comparisons of assimilatory nitrate reductases. Plant Physiol. 1981 Dec;68(6):1226–1230. doi: 10.1104/pp.68.6.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P. Purification of NADH-Nitrate Reductase by Affinity Chromatography. Plant Physiol. 1975 Dec;56(6):853–855. doi: 10.1104/pp.56.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]