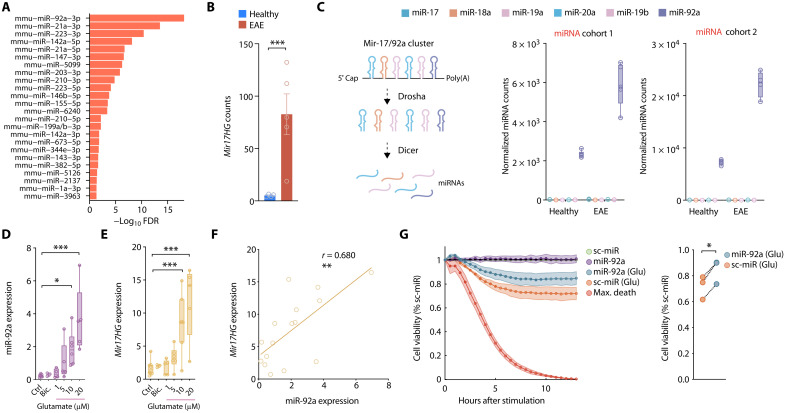
Fig. 2. Neuroprotective miR-92a is induced by neuroinflammation and excitotoxicity.
(A) Motor neuronal miRNA candidates identified across the two independent EAE cohorts (miRNA cohort 1: n = 5 per group; miRNA cohort 2: n = 4 per group) ranked by statistical significance. (B) Normalized Mir17HG seq-counts of spinal cord motor neurons after GFP-RPL10A IP (TRAP) from healthy and acute EAE (day 15 after immunization) Chat-EGFP/Rpl10a mice. (C) Scheme of miR-17/92a cluster (left) and normalized sequencing counts of cluster members (right) in motor neurons from healthy and acute EAE mice. (D) qRT-PCR of miR-92a (relative to sno234) in primary neurons 24 hours after stimulation with increasing concentrations of glutamate (Glu) or bicuculline (Bic.) (Ctrl, n = 8; Bic., n = 3; 1 μM Glu, n = 5; 5 μM Glu, n = 6; 10 μM Glu, n = 7; 20 μM Glu, n = 5). ROUT outlier analysis; one-way ANOVA, F5,24 = 7.870, P = 0.0002; Dunnett’s test. (E) qRT-PCR of Mir17HG (relative to Tbp) in primary neurons stimulated as in (D). One-way ANOVA, F5,28 = 10.59, P < 0.0001. (F) Pearson correlation of relative miR-92a and Mir17HG expression assessed by qRT-PCR 24 hours after exposure to different concentrations of glutamate (16 XY pairs, P = 0.0019). (G) Real-time viability assay 2 days after transfection of primary neurons with 25 nM miR-92a mimic or scrambled miRNA (sc-miR) control. Neurons were either stimulated with glutamate or vehicle control. Each well was normalized to its own baseline luminescence after 5 hours, to the unstimulated sc-miR control (100% cell viability), and to the max death control (5 mM glutamate, 0% cell viability); n = 3; paired t test, P = 0.011.