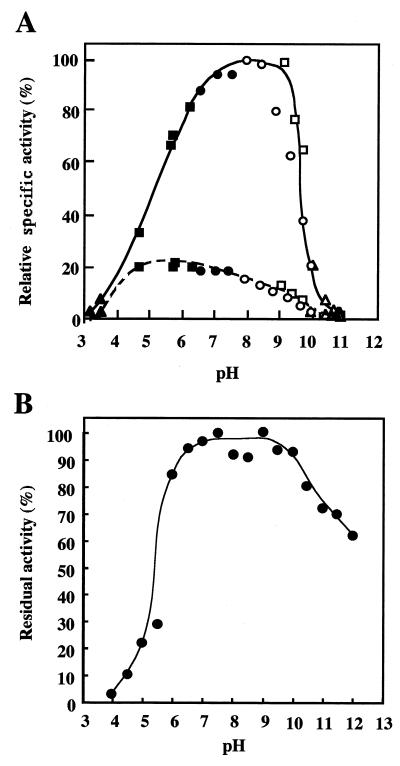
FIG. 2.
Effect of pH on the activity and stability of LAMY. (A) The pH activity curves of purified LAMY and BLA (each at 0.2 U ml−1) are shown by solid and dotted lines, respectively. The buffers used (50 mM each) were as follows: glycine-HCl, pH 3.0 to 3.5 (▴); acetate, pH 4.0 to 6.0 (■); Tris-HCl, pH 6.5 to 8.5 (•); glycine-NaOH, pH 9.0 to 10.5 (□); glycine-NaCl-NaOH, pH 8.0 to 10.5 (○); and carbonate, pH 10.0 to 11.0 (▵). The values are shown as percentages of the maximum specific activity of LAMY observed at pH 8.0 to 8.5, which is taken as 100%. (B) To assess the pH stability of LAMY, the enzyme (2.0 U ml−1) was preincubated at the indicated pH in 10 mM Britton-Robinson buffer and at 40°C for 30 min, and then samples (0.1 ml) were used for the measurements of the residual activity under the standard conditions of the enzymatic assay. The values are shown as percentages of the original activity, which is taken as 100%.