Figure 4.
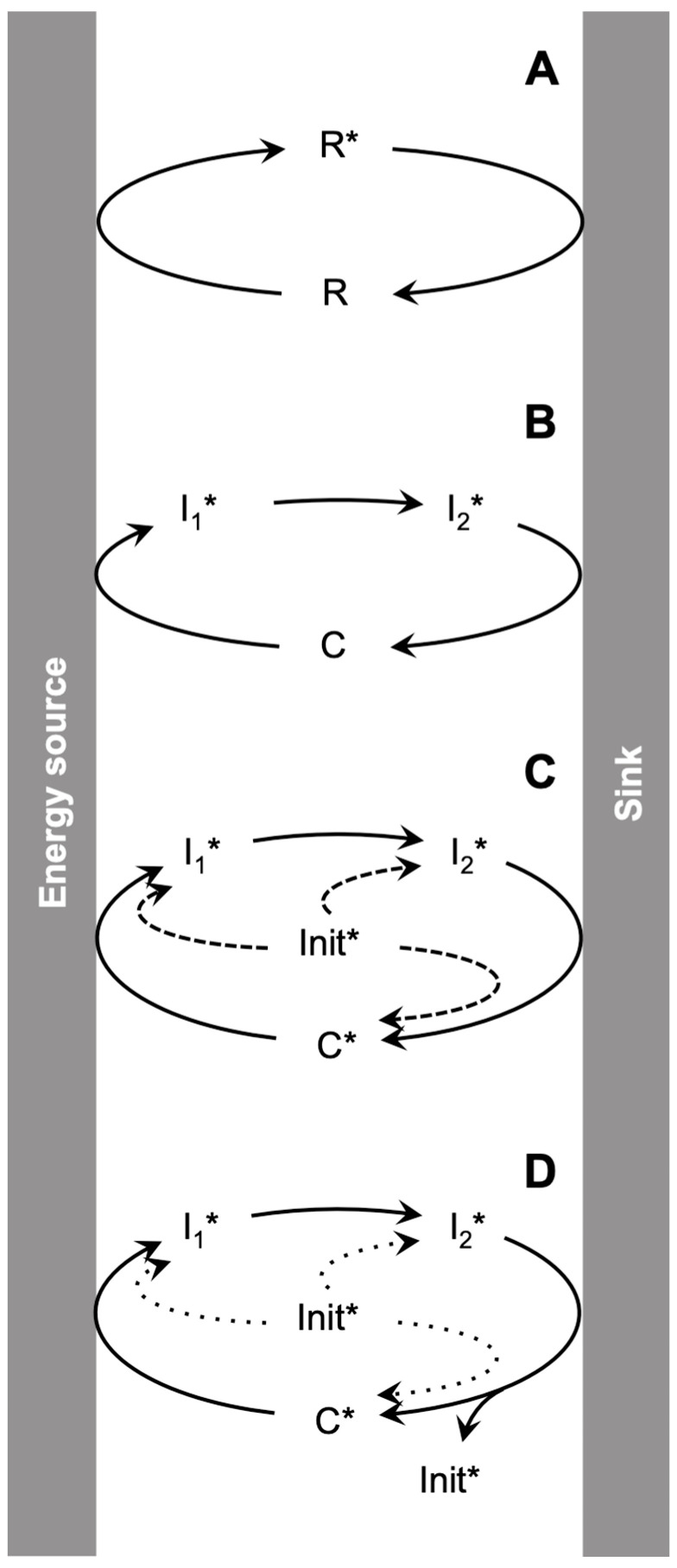
Dissipation associated with chemical processes of increasing complexity occurring in a disequilibrium context involving an energy source and a sink (for simplicity, the addition of reactants and the release of inactivated products have been omitted from this scheme and can be considered as belonging, respectively to the source or the sink). (A) The activation of a reagent R can lead to the thermodynamically unstable form R* in a kinetically stable state that reverts to the reactant by releasing its energy content as heat. Apart from its energy content, chemical work can be produced and stored in the system only if the activated form R* is specifically subject to an additional process such as polymerization (dissipative self-assembly). (B) Process involving a chemically stable catalyst C, which, in principle, can be totally regenerated through a catalytic cycle. The activated intermediates I1* and I2* are formed in a DKS state with the cleavage of these species releasing heat, inactivated products and regenerating the stable catalyst C. The whole system cannot be considered as being in a DKS state since C corresponds to a ground state and energy is stored in the system only to maintain the DKS concentration of intermediates I1* and I2*. (C) Process involving an unstable catalyst C*. In this case, the usual non-quantitative character of chemical reactions would lead to a breakdown of the catalyst leading to limited turnover numbers except if an energized (activated) initiator Init* is continuously provided into the system. Init* can in principle be any one of the components of the catalytic cycle, C*, I1* or I2* since reconstituting the whole cycle needs only one of its components. Interestingly, differentiating the initiator from the catalyst illustrates the fact that most of the energy stored in the process does not mandatorily come from the energy source but also from the activated initiator Init*. (D) A self-maintained catalytic system corresponding to the definition of an Emerging Autonomous System (EAS) in which the initiator Init* is produced in one extra copy through a reaction cycle which is effectively autocatalytic. In this case, the whole catalytic cycle (including C*, I1* and I2*), rather than specific individual intermediates, becomes a rudimentary autonomous system (at least autonomous from the initiator) and is both DKS-stable and able to store energy derived from some source and to grow at its expense in a way similar to the cell cycle of Figure 2. Interestingly, the activated initiator Init* constitutes a true initiator that needs only to be added once, though the entire system would end up collapsing if the non-equilibrium environment was to dissipate. Restarting the system would then require a new initiation using the activated initiator Init*.