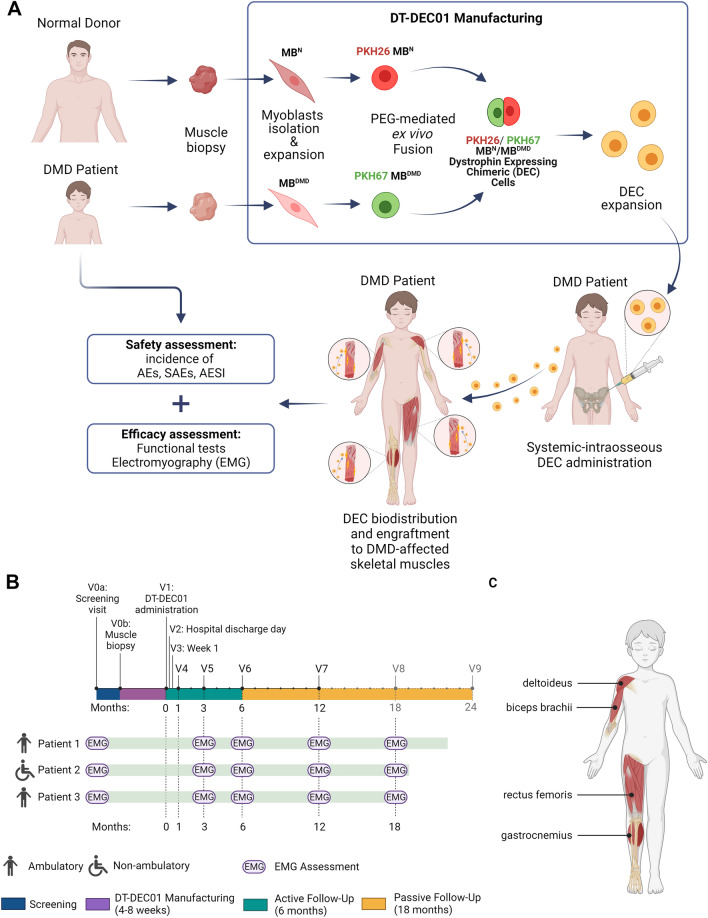
Fig. 1.
The outline of the first-in-human pilot study assessing safety and efficacy of the systemic–intraosseous administration of DT-DEC01 therapy in DMD patients. A Manufacturing of DT-DEC01 begins with muscle biopsies harvested from the DMD patient and the normal donor, followed by myoblasts isolation and expansion, PKH staining and PEG-mediated fusion creating DEC cells, followed by DEC sorting, expansion, product formulation and DT-DEC01 administration to DMD patient. B The timeline of EMG parameters assessment of MUP duration and amplitudes in the selected muscles (deltoideus, biceps brachii, rectus femoris and gastrocnemius) of DMD patients at the scheduled visits of: V0a screening visit, V0b skeletal muscle biopsy of DMD patient and the normal donor, V1 intraosseous DT-DEC01 administration. Active follow-up of 6 months after DT-DEC01 administration: V2 hospital discharge day, V3 week 1, V4 month 1, V5 month 3, V6 month 6. Passive follow-up of 18 months after DT-DEC01 administration: V7 month 12, V8 month 18, V9 month 24, EMG electromyography assessment. C Selected muscles of DMD patients assessed by EMG. Upper extremity: deltoideus muscle and biceps brachii; lower extremity: rectus femoris and gastrocnemius muscle. Figure created with BioRender.com