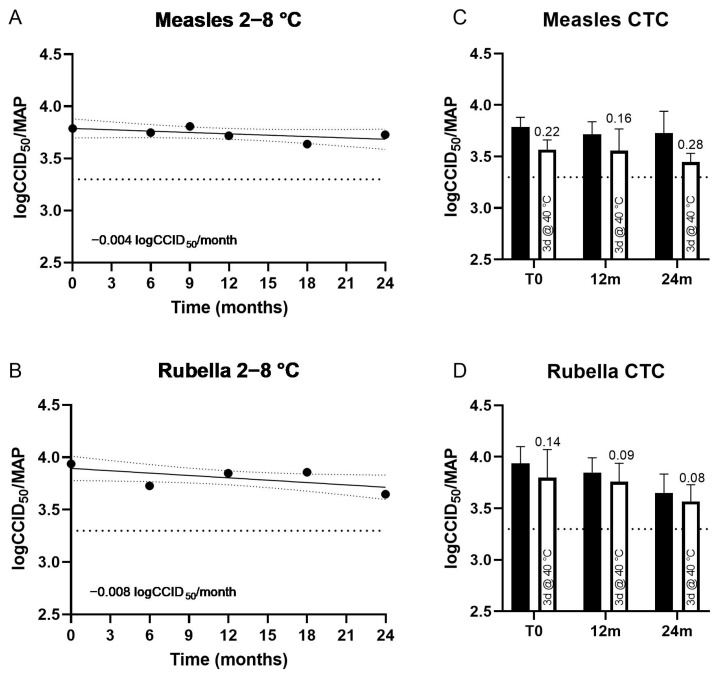
Figure 3.
Long-term thermostability (potency) at 2–8 °C of measles (A) and rubella (B) on measles and rubella (MR) HD-MAPs used for clinical study. At nine months, rubella testing did not meet assay validity criteria, and an insufficient number of HD-MAPs were available to repeat testing. Linear regression was performed, and 95% confidence bands are shown. Stability of measles (C) and rubella (D) after 3 days at 40 °C following prior long-term storage at 2–8 °C for 12 months and 24 months (white bars), compared with HD-MAPs stored at 2–8 °C only (black bars). At the initial time point (T0), 12 months, and 24 months, five HD-MAPs were stored at 40 °C, 60–75% relative humidity (RH) for three days prior to testing (white bar), then assayed for measles (C) and rubella (D) potency in parallel with HD-MAPs stored at 2–8 °C (black bar). The average log loss relative to HD-MAPs stored at 2–8 °C and assayed in parallel is shown above the bar, bars represent 95% confidence interval. For all graphs, minimum specification (3.3 logCCID50 per virus per HD-MAP) is shown as a dotted line.