Table 2.
Synthesis of arylpyrrolenitriles.
| Raw Materials | Reaction Equation |
|---|---|
| α-P-chlorophenylglycine |
|
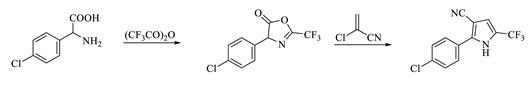
| P-chlorophenylglycine and TFAA after lactonization and pyrrole cyclization to obtain arylpyrrolenitrile [2,38,39]. | |
| |
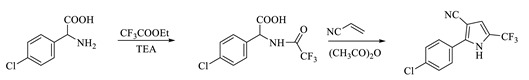
| P-chlorophenylglycine triethylamine and TFAE after condensation and pyrrole cyclization to obtain arylpyrrolenitrile [40]. | |
| |
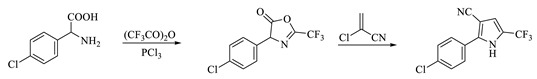
| P-chlorophenylglycine and TFAA with phosphorus trichloride as the catalytic agent after lactonization and pyrrole cyclization to obtain arylpyrrolenitrile [36]. | |
| |
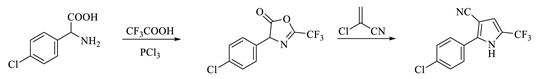
| P-chlorophenylglycine and TFA with phosphorus trichloride as the catalytic agent after lactonization and pyrrole cyclization to obtain arylpyrrolenitrile [37]. | |
| P-chlorobenzonitrile |
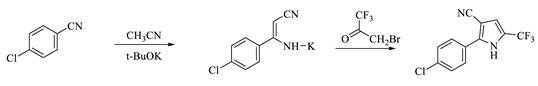
|
| P-chlorobenzonitrile, ethylene glycol dimethyl ether, dimethyl ether, acetonitrile, and potassium tert-butoxide after heating under reflux to obtain the intermediate product. Then, it reacts with 3-bromo-trifluoroacetone to getting arylpyrrolenitrile [41]. | |
| P-chlorobenzyl chloride |
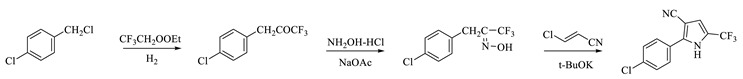
|
| P-chlorobenzyl chloride is added to an ether solution with magnesium flakes and TFAE after the reaction to obtain intermediate product 1. Then, it reacts with hydroxylammonium chloride and sodium acetate to obtain intermediate product 2. Finally, potassium tert-butoxide and β-chloroacrylonitrile are used to obtain arylpyrrolenitrile [42]. | |
| P-chlorobenzylamine |
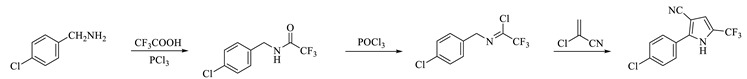
|
| P-chlorobenzylamine and TFA after acetylation, chlorination, and cycloaddition reaction in the presence of phosphorus trichloride to obtain arylpyrrolenitrile [43,44]. | |
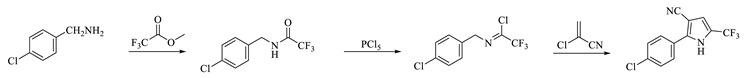
| |
| P-chlorobenzylamine and methyl trifluoroacetate after reaction in the presence of methanol, phosphorus pentachloride, and acetonitrile to obtain arylpyrrolenitrile [45]. | |
| P-chlorobenzoyl chloride |
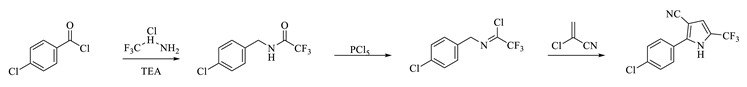
|
| P-chlorobenzoyl chloride and triethylamine after acetylation, chlorination, and cycloaddition reaction in the presence of phosphorus trichloride to obtain arylpyrrolenitrile [46,47]. | |
| P-chlorophenylamine acrylonitrile |
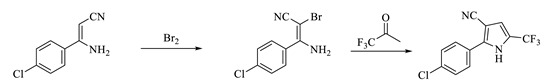
|
| Bromine dissolved in carbon tetrachloride reacts with p-chlorophenylaminoacrylonitrile to obtain α-bromo-p-chloro-β-aminoacrylonitrile. Then, it reacts with trifluoroacetone in the presence of acetic acid to obtain arylpyrrolonitrile [35,48]. | |
| α-P-chlorophenyl trifluoroacetamcinonitrile |
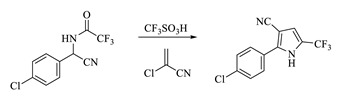
|
| α-P-chlorophenyl trifluoroacetylaminonitrile dissolved in toluene reacts with trifluoromethanesulfonic acid to obtain the intermediate product. Then, after the reaction with 2-chloroacrylonitrile, arylpyrrolonitrile is obtained [49]. |
