This editorial refers to ‘Inhibition of galectin-3 post-infarction impedes progressive fibrosis by regulating inflammatory profibrotic cascades’, by X. Wang et al., https://doi.org/10.1093/cvr/cvad116.
The adult mammalian heart has negligible regenerative capacity; thus, repair after myocardial infarction requires formation of a collagen-based scar mediated through sequential activation of inflammatory and fibrogenic pathways. Although reparative fibroblasts protect the infarcted heart from catastrophic rupture and dilative remodelling, pro-fibrotic signalling needs to be tightly regulated to prevent exaggerated fibrosis and heart failure progression. In the infarcted heart, both defective and excessive activation of fibroblasts can be detrimental. Thus, clinical implementation of anti-fibrotic strategies in myocardial infarction is challenging and requires not only dissection of pathways mediating fibroblast activation but also identification of patients with exaggerated, unrestrained, or prolonged fibrogenic responses.1
Galectin-3 is a β-galactoside-binding lectin with an important role in regulation of inflammation, repair, and fibrosis. A large body of evidence suggests that circulating galectin-3 levels may contribute important prognostic information in patients with myocardial disease, predicting adverse outcome in patients with myocardial infarction2 or heart failure.3 In addition to its role as a biomarker, galectin-3 may be also causally involved in the pathogenesis of cardiac remodelling. Myocardial galectin-3 expression is markedly and consistently upregulated in experimental models of myocardial infarction and heart failure and is predominantly localized in activated macrophages.4 Some, but not all studies have suggested a central role for galectin-3 in the pathogenesis of adverse cardiac remodelling, predominantly mediated through fibroblast-activating effects.5 Whether these fibrogenic effects can be targeted therapeutically in myocardial infarction to selectively inhibit maladaptive fibrotic remodelling without affecting the reparative response is unknown.
In the current issue of the journal, Wang et al.6 examined the effects of pharmacologic galectin-3 inhibition in experimental myocardial infarction. The authors showed that a 7-day course of truncated C-terminal galectin-3 (Gal-3C), which acts as a dominant negative galectin-3 inhibitor, protected the infarcted heart from adverse remodelling and dysfunction in both reperfused and non-reperfused rat models of myocardial infarction, when initiated 4 days after coronary occlusion. The protective effects of Gal-3C treatment were associated with attenuated peri-infarct interstitial fibrosis and were attributed to modulation of macrophage phenotype, resulting in decreased secretion of macrophage-derived fibrogenic mediators. Although the translational value of the findings is somewhat diminished by the exclusive focus on male animals, the authors performed a very systematic translationally relevant study that included experiments identifying the optimal dose of Gal-3C, investigations examining the impact of aging on the effectiveness of the strategy, and a comparison with the protective effects of established therapeutic agents with known anti-fibrotic actions, such as angiotensin receptor blockers (ARB) and mineralocorticoid receptor antagonists (MRA). Much like young animals, old rats also exhibited attenuated dysfunction and reduced adverse remodelling upon Gal-3C treatment. Moreover, galectin-3 inhibition was more effective than the ARB losartan, or the MRA spironolactone in attenuating post-infarction systolic dysfunction. Overall, the investigation represents an important step towards translation of galectin-3 targeting approaches in myocardial infarction. In addition, the study also raises several important questions regarding the cellular targets of galectin-3 in infarcted hearts and the potential risks of galectin-3 inhibition, and highlights a promising opportunity for biomarker-guided therapy to attenuate post-infarction fibrosis and adverse remodelling.
1. What are the cellular targets of galectin-3 in the infarcted myocardium?
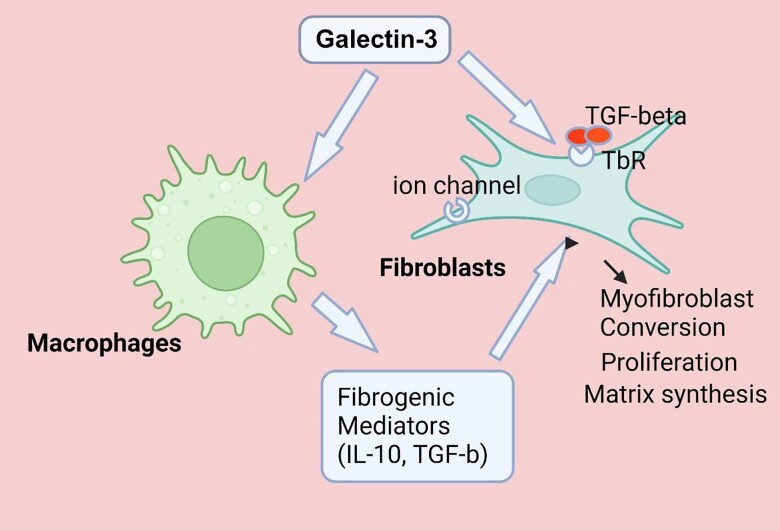
In addition to its effects in stimulating a fibrogenic macrophage phenotype, galectin-3 may promote adverse remodelling through actions on other cell types. The direct effects of galectin-3 on fibroblasts may be particularly important in the infarcted myocardium (Figure 1). Galectin-3 potently activates fibroblasts through several distinct mechanisms. First, galectin-3 may interact with cell surface glycoconjugates, activating mitogenic signalling cascades and promoting fibroblast proliferation.7 Second, galectin-3 may augment Transforming Growth Factor (TGF)-β receptor function,8 accentuating TGF-β-mediated myofibroblast activation. Third, galectin-3 has been suggested to act by stimulating KCa3.1 ion channels, resulting in hyperpolarization and a subsequent increase in Ca2+ entry in fibroblasts, that may mediate their activation.9 Additional effects of galectin-3 on other cell types, such as stimulation of endothelial to mesenchymal transition and activation of pro-apoptotic pathways in cardiomyocytes may also contribute to adverse remodelling of the infarcted heart; however, their in vivo role has not been convincingly documented.
Figure 1.
The fibrogenic effects of galectin-3 in myocardial infarction may involve actions on macrophages and fibroblasts. Galectin-3 stimulates an M2-like phenotype in macrophages, stimulating synthesis of fibroblast-activating mediators, such as IL-10 and TGF-βs. Fibrogenic effects of galectin-3 may also involve direct actions on fibroblasts, increasing proliferation, stimulating extracellular matrix synthesis, and mediating myofibroblast conversion. Interactions between galectin-3 and glycoconjugates on the fibroblast surface may accentuate growth factor signalling. Moreover, effects of galectin-3 on ion channels may stimulate Ca2+ entry, thus promoting fibroblast activation. TbR, TGF-β receptor complex. Created with biorender.com.
2. Could galectin-3 inhibition perturb repair of the infarcted heart?
Considering the central role of fibroblasts in repair of the infarcted heart, implementation of anti-fibrotic strategies in myocardial infarction may carry significant risks. Overzealous or non-selective suppression of infarct fibroblast activity can cause cardiac rupture, or accentuate dilative remodelling due to perturbations in the structure of the collagenous network that may reduce the tensile strength of the scar. Whether galectin-3 inhibition is fraught with similar risks likely depends on the timing of treatment and the magnitude of the inhibitory effect. Wang et al. did not report dose-dependent adverse effects of Gal-3C treatment. Moreover, treatment prior to infarction was not associated with an increased incidence of rupture. However, in a study using galectin-3 knockout mice, global germline loss of galectin-3 increased infarct-related mortality and exaggerated adverse post-infarction remodelling.10 The contrasting effects of galectin-3 disruption in the genetic and pharmacologic targeting studies may reflect a requirement for basal galectin-3 signalling for repair of the infarcted heart, and partial inhibitory actions of Gal-3C treatment that may preserve the reparative functions of galectin-3 signalling.
3. Do the animal model findings predict therapeutic efficacy of Gal-3C treatment in patients with myocardial infarction?
Animal models have significant limitations. Although well suited to address questions on cell biological mechanisms and molecular pathways, animal models cannot recapitulate the pathophysiologic heterogeneity of the human pathologic condition. In patients with myocardial infarction, fibrosis, remodelling and dysfunction are dependent not only on the severity of the initial injury but also on a broad range of other factors, such as the genetic profile of the patients, comorbid conditions (such as aging, diabetes, and dyslipidaemias), patterns of atherosclerotic disease, and the effects of medications. Despite the authors’ conscientious efforts to examine the impact of aging on therapeutic efficacy, and to compare the effects of Gal-3C treatment with those of ARBs and MRAs, only rigorous clinical investigations can provide information on the effectiveness of galectin-3 inhibition in patients with myocardial infarction. Considering the heterogeneity of reparative and fibrotic responses in the clinical context, galectin-3 may be beneficial only in a sub-population of myocardial infarction patients that exhibit overactive fibrotic responses.
4. An opportunity for biomarker-guided therapy
The extensive evidence on the role of circulating galectin-3 as a prognostic biomarker that may reflect cardiac fibrogenic activation, and the findings of the current study demonstrating protective effects of galectin-3 inhibition in post-infarction remodelling generate substantial enthusiasm regarding the potential for clinical implementation. Assessment of galectin-3 levels in patients surviving acute myocardial infarction may serve to identify subjects with exaggerated galectin-3-driven fibrosis that may benefit from treatment with Gal-3C, while sparing those with low galectin-3 levels from the potentially deleterious effects of anti-fibrotic therapy. Thus, galectin-3 targeting represents a highly promising opportunity for a biomarker-guided approach to prevent excessive fibrosis and to protect from adverse remodelling in patients with myocardial infarction.
Funding
Dr Frangogiannis’ laboratory is supported by National Institutes of Health grants R01 HL76246, R01 HL85440, and R01 HL149407, and by U.S. Department of Defense grant PR211352.
References
- 1. Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res 2021;117:1450–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Tano G, Caretta G, De Maria R, Parolini M, Bassi L, Testa S, Pirelli S. Galectin-3 predicts left ventricular remodelling after anterior-wall myocardial infarction treated by primary percutaneous coronary intervention. Heart 2017;103:71–77. [DOI] [PubMed] [Google Scholar]
- 3. van der Velde AR, Gullestad L, Ueland T, Aukrust P, Guo Y, Adourian A, Muntendam P, van Veldhuisen DJ, de Boer RA. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail 2013;6:219–226. [DOI] [PubMed] [Google Scholar]
- 4. Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andreé S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004;110:3121–3128. [DOI] [PubMed] [Google Scholar]
- 5. Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, van Veldhuisen DJ, Bank RA, van Gilst WH, Silljé HHW, de Boer RA. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail 2013;6:107–117. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Gaur M, Mounzih K, Rodriguez HJ, Qiu H, Chen M, Yan L, Cooper BA, Narayan S, Derakhshandeh R, Rao P, Han DD, Nabavizadeh P, Springer ML, John CM. Inhibition of galectin-3 post-infarction impedes progressive fibrosis by regulating inflammatory profibrotic cascades. Cardiovasc Res 2023;119:2536–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inohara H, Akahani S, Raz A. Galectin-3 stimulates cell proliferation. Exp Cell Res 1998;245:294–302. [DOI] [PubMed] [Google Scholar]
- 8. Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, Simpson AJ, Forbes SJ, Hirani N, Gauldie J, Sethi T. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med 2012;185:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. She G, Hou MC, Zhang Y, Zhang Y, Wang Y, Wang HF, Lai BC, Zhao WB, Du XJ, Deng XL. Gal-3 (Galectin-3) and K(Ca)3.1 mediate heterogeneous cell coupling and myocardial fibrogenesis driven by βAR (β-Adrenoceptor) activation. Hypertension 2020;75:393–404. [DOI] [PubMed] [Google Scholar]
- 10. Cassaglia P, Penas F, Betazza C, Fontana Estevez F, Miksztowicz V, Martinez Naya N, Llamosas MC, Noli Truant S, Wilensky L, Volberg V, Cevey AC, Touceda V, Cicale E, Berg G, Fernández M, Goren N, Morales C, González GE. Genetic deletion of galectin-3 alters the temporal evolution of macrophage infiltration and healing affecting the cardiac remodeling and function after myocardial infarction in mice. Am J Pathol 2020;190:1789–1800. [DOI] [PubMed] [Google Scholar]