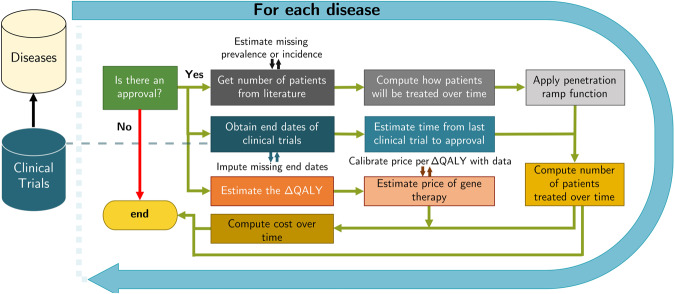
Fig. 1. A flowchart showing the performance of the simulation.
After extracting the information on each disease from the clinical trial databases, we simulate whether the disease will obtain an approval. If it fails to do so, the simulation will end for this disease in this iteration. Otherwise, we estimate the expected number of patients to be treated, compute the corresponding cost of treatment, and store the results. At each step of the computation, we sourced data from the published literature and impute missing information.