Abstract
Metaplastic breast cancer (MBC) is a rare subtype of invasive breast cancer characterized by mixed epithelial and mesenchymal differentiation. Commonly seen subtypes include squamous cell carcinoma, spindle cell carcinoma, and metaplastic carcinoma with heterologous mesenchymal differentiation. MBC tends to have a more aggressive clinical presentation, higher metastatic potential, higher rates of local recurrence, and a worse prognosis compared with invasive breast carcinoma of no special type. Most MBCs are triple-negative breast cancers, which explains their resistance to most systemic therapies. Therefore, accurately diagnosing MBC early is crucial for deciding the treatment strategy and predicting the prognosis. In this pictorial essay, the imaging findings of MBC in different modalities and the histopathologic features of its subtypes are reviewed.
Keywords: Metaplastic breast cancer, mammography, ultrasonography, magnetic resonance imaging, histopathology
Main points
• Metaplastic breast cancer (MBC) tends to demonstrate benign imaging features on mammography (MG) and ultrasound.
• A predominantly circumscribed high-density mass without calcifications on MG should raise suspicion of MBC.
• High T2-weighted signal intensity, rim enhancement, and non-enhancing internal components on magnetic resonance imaging help differentiate MBC from other breast malignancies.
• Squamous cell carcinoma tends to reveal an irregularly-shaped cystic mass with spiculated margins, while spindle cell carcinoma is more likely to show benign imaging features, such as an oval shape and circumscribed margins.
• Histopathologic evaluation, including immunohistochemistry analysis, is essential for diagnosing MBC.
Metaplastic breast cancer (MBC) is a rare subtype of invasive breast cancer that accounts for less than 1% of all breast cancers.1 Currently, the diagnosis of MBC is made more frequently as the histologic features are clearly defined, and the pathology methods have improved. Histologically, MBC is characterized by the presence of two or more malignant cell types, commonly a mixture of epithelial and mesenchymal components. According to the most recent World Health Organization classification of breast tumors, the histologic types of MBC are low-grade adenosquamous carcinoma, fibromatosis-like metaplastic carcinoma, spindle cell carcinoma, squamous cell carcinoma, metaplastic carcinoma with heterologous mesenchymal differentiation, and mixed metaplastic carcinoma.2 In a large international multicenter study of 405 patients with histologic confirmation of MBC, spindle cell (34%) was the most common subtype in the Western series, and squamous cell (34%) was the most common subtype in the Asian series, followed by metaplastic carcinoma with heterologous mesenchymal differentiation in both series (29% and 24%, respectively).3
MBC usually presents as a fast-growing large palpable mass in women older than 50 years, with no predilection for the left or right breast,4 and it tends to metastasize hematogenously rather than through the lymphatic system.5 This is why axillary lymph node metastasis is rare, while the lungs and bones are the most common sites for MBC to metastasize, which may explain why patients usually present in the advanced stages more frequently than with invasive breast carcinoma of no special type (IBC-NST).
The differential diagnosis between MBC and IBC-NST is important for the clinical management, treatment strategy, and prognosis prediction.6 MBC tends to have a more aggressive clinical presentation, higher metastatic potential, higher rates of local recurrence, and worse prognosis compared with IBC-NST.7 The majority of MBC is triple-negative breast cancer, in which estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 are negative. Triple-negative breast cancers are resistant to chemotherapy, hormonal therapy, and targeted molecular therapy, which explains the poor prognosis and the challenging clinical management for patients with MBC.8,9 Therefore, accurately diagnosing MBC at an early stage is crucial for survival. This article aims to review the imaging findings of MBC in different modalities and describe the histopathologic features of its subtypes.
Imaging findings
Mammography (MG) findings
In MG examinations, MBCs tend to present with benign imaging features; these cancers are commonly hyperdense lesions with round (Figure 1) or oval (Figures 2, 3) shapes.10,11 The margins of these lesions may be circumscribed (Figure 1), obscured (Figures 3, 4), or indistinct (Figures 5, 6).1,10,11 Partially circumscribed margins are a frequently encountered and possible distinctive imaging feature, reflecting the presence of both the metaplastic and invasive carcinoma components.1 An irregular shape (Figure 6) and spiculated margins are uncommon imaging features in MBCs.6,10,12 Additionally, MBCs are generally large tumors with a mean size of 3.9 cm according to the cases reviewed (Figures 1, 2).13,14 One study reported that 20.4% of MBCs were larger than 5 cm, compared with only 5.2% of invasive ductal carcinomas.15 Microcalcifications (Figure 6) are less common in MBCs than in IBC-NST.6 Calcifications are often absent in MBCs and are described in up to 25% of cases, with greater frequency in matrix-producing subtypes, such as carcinomas with chondroid metaplasia.16 If present, they can be amorphous, coarse, punctate, or pleomorphic.
Figure 1.
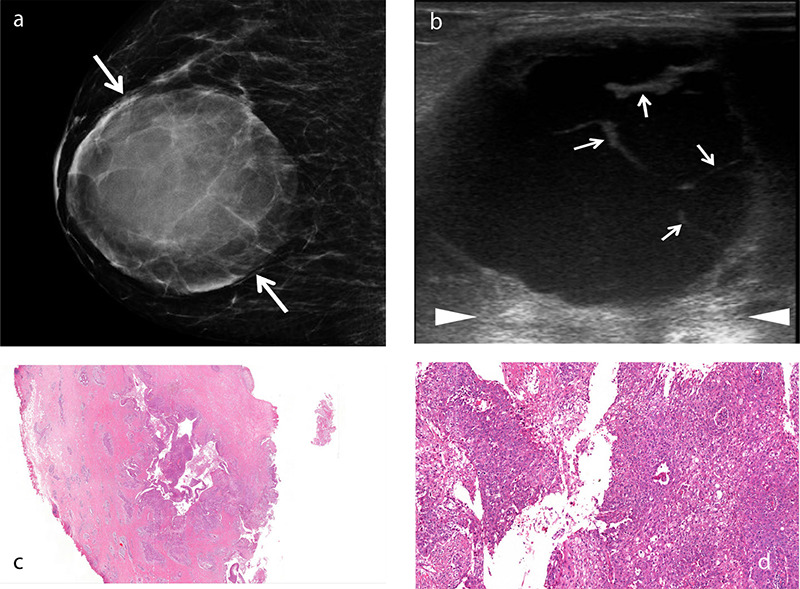
An example of squamous cell metaplastic breast carcinoma with extensive central necrosis. A 38-year-old female patient presented with a rapidly growing palpable mass in the right breast. Mammogram (a) reveals a large, well-defined round density mass measuring 8 × 7.5 cm with circumscribed margins (arrows). Gray-scale ultrasound image (b) shows a complex cystic mass with thick septa (b, arrows) and posterior acoustic enhancement (arrowheads). Histopathology of the cystic lesion (c) reveals atypical squamous cells lining the cavity and infiltrating the adjacent stroma [hematoxylin and eosin (H&E), ×2]. Photomicrograph (d) shows that the neoplastic cells lining the irregular spaces are atypical squamous cells with abundant eosinophilic cytoplasm and large, vesicular nuclei (H&E, ×20).
Figure 2.
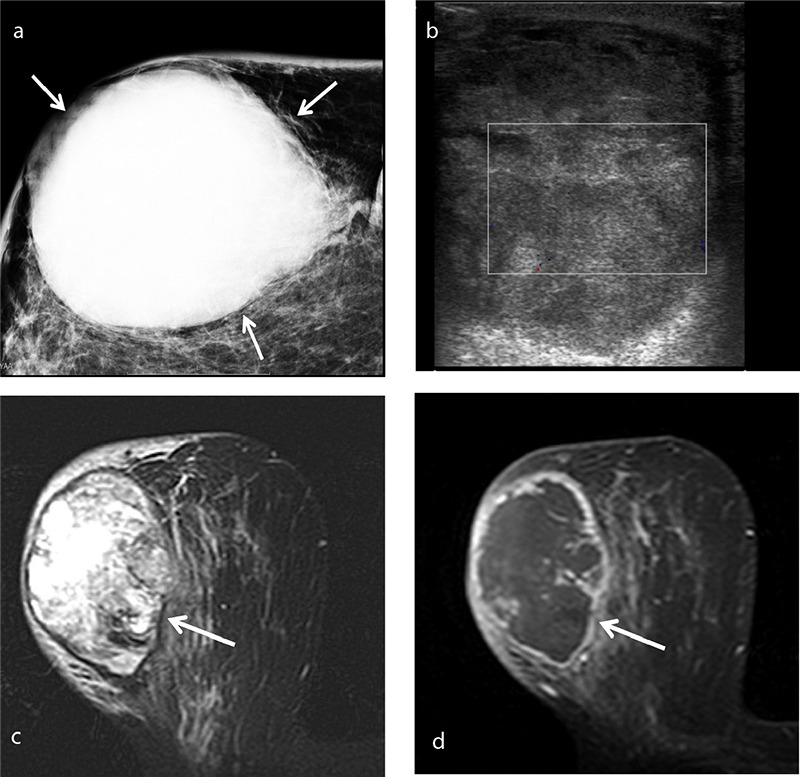
An example of spindle cell type metaplastic breast carcinoma in a 70-year-old female patient who presented with a palpable mass in her right breast. Mammogram (a) shows a high-density oval mass measuring 7.5 × 6 cm with obscured margins (arrows). Color Doppler ultrasound (b) shows a lesion adjacent to the skin with a heterogeneous echo pattern and no significant internal vascularity. T2-weighted magnetic resonance imaging (c) reveals a mass with heterogeneous intermediate-to-high signal intensity. Axial subtracted contrast-enhanced T1-weighted image (d) shows a rim-enhancing lesion (arrow) with a large non-enhancing central area consistent with necrosis, corresponding to the avascular area on the Doppler ultrasound image.
Figure 3.
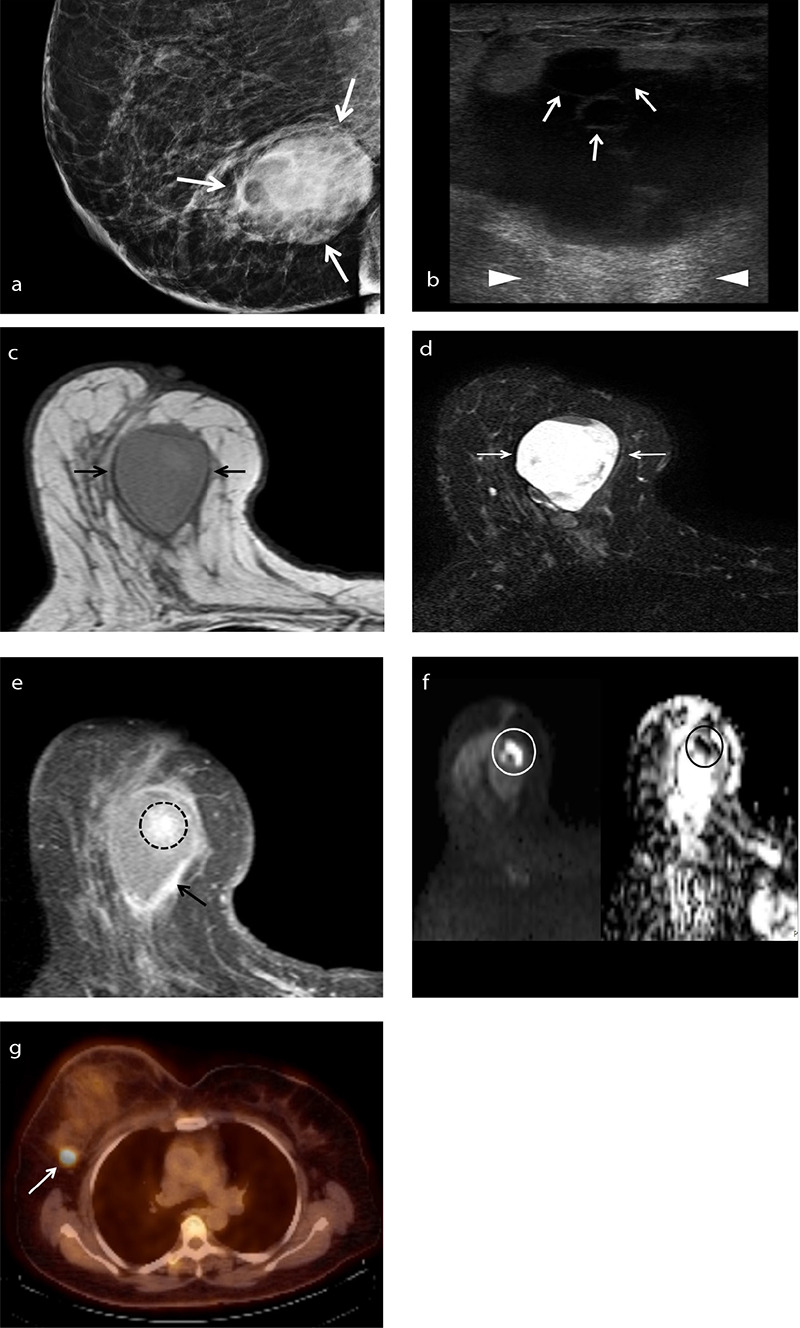
An example of squamous cell metaplastic breast carcinoma in a 58-year-old female patient who presented with a palpable mass in her right breast. Mammogram (a) shows a large high-density mass measuring 4.7 × 3.8 cm with obscured margins (arrows). Gray-scale ultrasound image (b) shows a cystic mass with septa (arrows) and posterior acoustic enhancement (arrowheads). T1-weighted magnetic resonance image (c) shows an oval lesion with circumscribed margins and isointense relative to surrounding fibroglandular tissue. Axial fat-suppressed T2-weighted magnetic resonance image (d) shows a heterogeneous high signal intensity mass. T1-weighted fat-suppressed post-contrast image (e) shows an irregular enhancing rim (arrow) at the tumor periphery and a central area of heterogeneous enhancement (dashed circle). Diffusion-weighted imaging (f) reveals that the central contrast-enhancing area restricts diffusion (circles, left: diffusion-weighted image, right: corresponding apparent diffusion coefficient map). Axial fused fluorodeoxyglucose positron emission tomography/computed tomography image (g) shows the right axillary lymph node with intense fluorodeoxyglucose uptake (standardized uptake value max: 5.3, arrow), consistent with metastasis.
Figure 4.
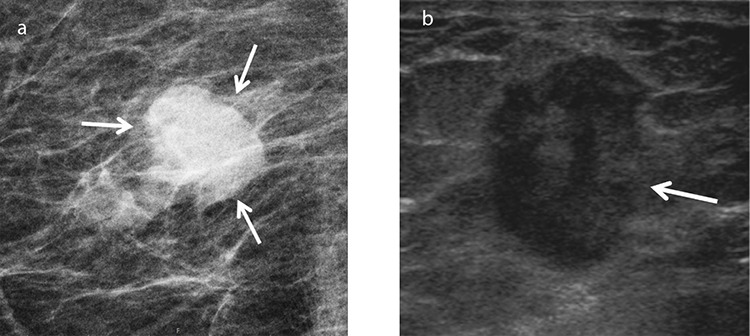
An example of chondroid matrix-producing metaplastic breast carcinoma in a 77-year-old female patient who presented with a palpable lump in her right breast. Mammogram (a) shows a density with obscured margins (arrows). Gray-scale ultrasound (b) shows an oval, vertically oriented, hypoechoic lesion measuring 1.3 × 1.8 cm with indistinct borders (arrow) and posterior acoustic enhancement.
Figure 5.
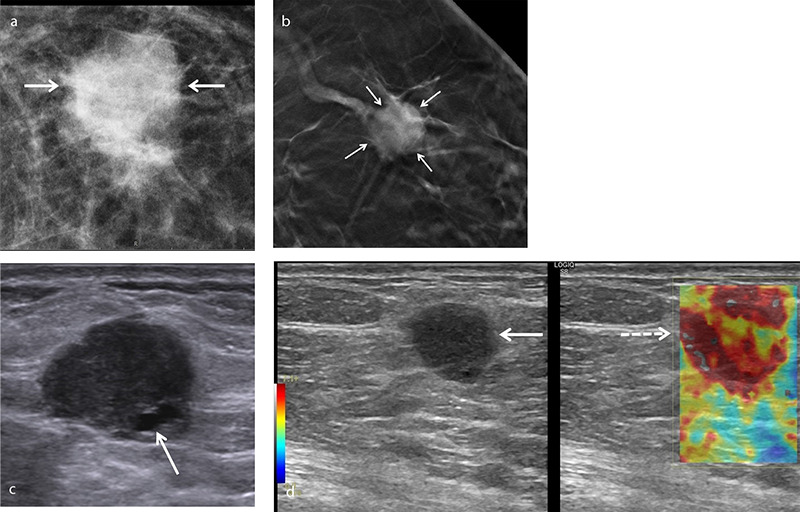
An example of squamous cell metaplastic breast carcinoma in a 51-year-old asymptomatic female patient. Mammogram (a) shows a round opacity with indistinct margins. Digital breast tomosynthesis (b) better demonstrates architectural distortion caused by the lesion (arrows). Gray-scale ultrasound (c) shows a round hypoechoic lesion measuring 1.8 × 1.4 cm with microlobulated margins and a small cystic area (arrow). Shear wave elastography image (d) reveals increased stiffness within and around the lesion.
Figure 6.
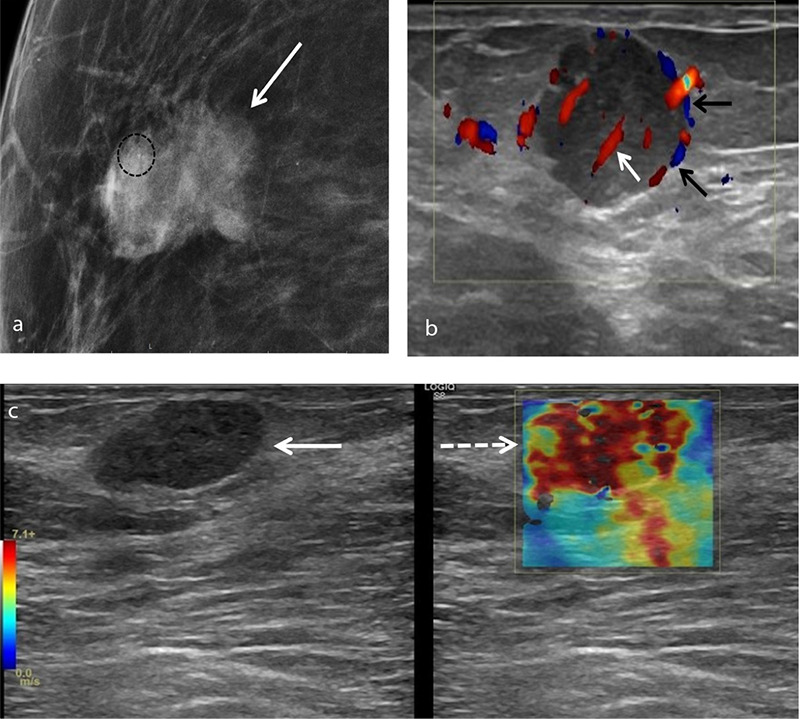
An example of squamous cell metaplastic breast carcinoma in a 72-year-old female patient who presented with a palpable superficial right breast mass. Mammogram (a) shows an irregularly shaped density (arrow) with indistinct margins and microcalcifications (circle). Color Doppler ultrasound (b) reveals an oval hypoechoic lesion measuring 2.3 × 1.9 cm with microlobulated margins, showing both peripheral (black arrows) and central (white arrow) vascularity. On shear wave elastography (c), the lesion displays increased stiffness compared to the surrounding fibroglandular tissue.
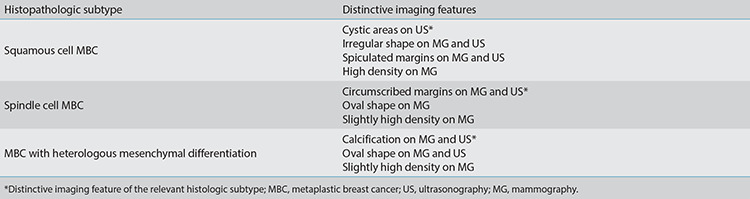
Different histologic subtypes of MBC may present with distinctive MG findings. Squamous cell carcinoma usually presents as an irregularly shaped mass (Figure 6) with spiculated margins and high density, while spindle cell carcinoma and matrix-producing carcinoma tend to present as an oval-shaped mass with circumscribed margins and slightly high density (Figure 2).17 Spindle cell carcinoma usually demonstrates benign imaging features on MG since it is surrounded by a fibrous capsule.18 Microplastic breast cancer lesions with heterologous mesenchymal differentiation are more likely to be calcified. A circumscribed margin with a spiculated portion is often seen in MBC with a mixture of metaplastic and invasive carcinoma.1
Ultrasonography (US) findings
On US, MBC is frequently encountered as a mass with a complex echo structure containing a cystic component secondary to necrosis (Figures 1, 3), although it can also be detected as a solid mass (Figure 4).1,6,12 In one study, central necrosis constituting more than 50% of the tumor was observed in 66% of patients.4 The shape of the lesions may be round, oval, or irregular. In addition, MBCs can have circumscribed (Figure 1), indistinct (Figure 4), or microlobulated (Figure 7) margins.1,6,12 Benign US imaging findings are more common in MBC than in IBC-NST,6 and MBCs often demonstrate posterior acoustic enhancement (Figures 1, 3) rather than posterior acoustic shadowing, making their differentiation from benign lesions even more challenging.6 Commonly detected US findings of MBC are round or oval-shaped lesions with well-defined margins, complex echogenicity, and posterior acoustic enhancement.12,16
Figure 7.
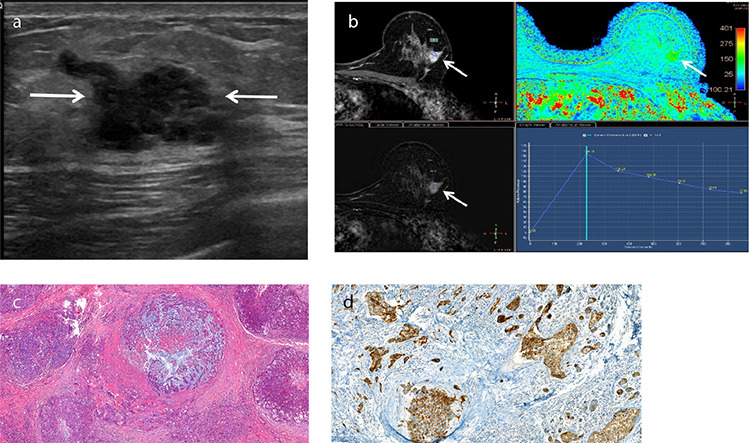
An example of metaplastic breast carcinoma with heterologous mesenchymal (chondroid) differentiation in a 43-year-old female patient who presented with a palpable mass in her left breast. Metaplastic breast carcinoma with heterologous mesenchymal (chondroid) differentiation. Gray-scale ultrasound (a) shows an irregularly shaped lesion measuring 2 × 1 cm with microlobulated and partially indistinct margins (arrows). T1-weighted dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) (b) demonstrates an irregularly shaped mass showing heterogeneous enhancement (arrows). Pharmacokinetic analysis of DCE-MRI (b, bottom-right) reveals a type three time-intensity curve. Photomicrograph (c) shows irregular nests of malignant epithelial cells are admixed with the chondroid component, which shows moderate pleomorphism (Hematoxylin and eosin, ×10). Immunohistochemical staining of high-molecular-weight cytokeratin (d) demonstrates cytoplasmic staining of the malignant epithelial cells (34βE12, ×20).
There are some US features that help to identify the subtypes of MBCs. Lesions with large cystic areas usually contain a squamous component (Figure 1).11 Similar to MG, spindle cell carcinoma tends to show benign imaging features on US, such as round or oval-shaped lesions with circumscribed margins (Figure 2). Rapidly growing high-grade MBCs are usually associated with ill-defined margins and less desmoplastic reaction at peritumoral sites.19
Routine B-mode ultrasound has long been the first-choice imaging guidance for breast biopsy as it is practical and effective. However, in the presence of hemorrhage or necrosis, the probability of misdiagnosis increases due to inadequate sampling or poor sampling site selection.11 Doppler US reveals the angioarchitecture of the lesions, allowing the differentiation of viable and necrotic portions (Figure 2). Therefore, adding color Doppler to routine B-mode US during the biopsy of these tumors would increase the chance of obtaining sufficient material for histopathologic examination.
Magnetic resonance imaging (MRI) findings
Breast MRI is a potential problem-solving tool with higher sensitivity and specificity in cases of ambiguous findings with conventional breast imaging modalities.20 On T1-weighted images, the signal of the MBC lesion is generally isointense or hypointense when compared with the normal fibroglandular tissue (Figure 3), similar to other histologic types of IBC.7 Heterogeneous high signal intensity on T2-weighted images is a significant MRI feature of MBC (Figures 2, 3).4,8 The T2 hyperintensity is related to the necrotic component of the tumor, a frequent finding in MBC. Therefore, the T2 hyperintensity helps distinguish MBC from other histologic types of invasive breast cancer, although differentiating from mucinous carcinoma and, less frequently, necrotic infiltrating ductal carcinoma is needed.4
The most common pattern of enhancement is a ring-like enhancement (Figures 2, 3), which can be partially explained by the extensive central necrosis observed in these tumors.10 Non-enhancing solid portions in the peripheral areas of the tumor might be explained by the presence of metaplastic tissue.8
Table 1 shows a comparison of the multimodality imaging features of MBC compared with IBC-NST. Table 2 shows the MG and US imaging characteristics of the major histologic subtypes of MBC.
Table 1. Comparison of multimodality imaging features of MBC compared with IBC-NST.
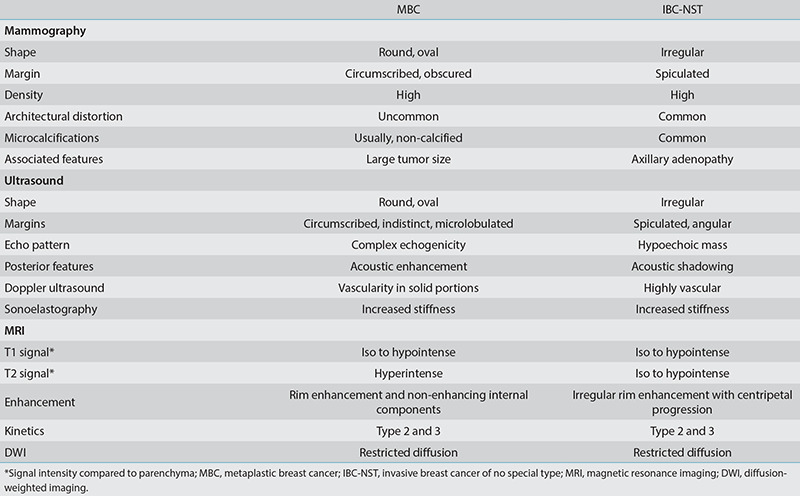
Table 2. Mammography and ultrasound imaging features of major histologic subtypes of MBC.
Advanced imaging techniques
Digital breast tomosynthesis (DBT) and contrast-enhanced MG are techniques that have the potential to improve the sensitivity of conventional MG. DBT significantly improves lesion detection by minimizing the effect of overlying breast tissue, and it can reveal architectural distortion better than conventional MG, particularly in dense breast tissue. Detection of architectural distortion on DBT (Figure 5) can be a valuable finding for diagnosing MBC, which tends to demonstrate benign imaging features.11 Contrast-enhanced MG is a novel technique that visualizes neovascularity using intravenous iodinated contrast material.
Sonoelastography is an imaging technique developed to improve the specificity of B-mode US, which reveals the relative stiffness of the target tissue compared to the surrounding tissue. Stiff breast lesions have a higher risk of being malignant. Based on our experience, MBC tends to reveal increased stiffness in shear wave sonoelastography (Figures 5, 6).
Diffusion-weighted imaging (DWI) and dynamic contrast-enhanced MRI are advanced MRI techniques commonly used in breast imaging. MBCs usually show restricted diffusion on DWI (Figure 3), which can be a valuable finding in differentiating MBCs from benign lesions. In a retrospective study including nineteen patients with MBC, DWI showed diffusion restriction in all cases.8 Early enhancement and delayed washout in the peripheral rim and non-enhancing internal components would be beneficial in distinguishing MBCs from other types of breast malignancies.7 The most frequent pharmacokinetic time–signal intensity curves are types two and three (Figure 7), which is not a specific characteristic of MBC.4
The high risk of early distant metastases in MBC necessitates systemic staging as part of the initial evaluation. Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) can detect metastases (Figure 3) that may not be visible on other imaging modalities. The specificity of FDG PET/CT is low within the breast as inflammatory causes, infections, benign tumors such as fibroadenomas, and even physiological conditions such as lactation may be markedly FDG avid.21 Other major roles of 18F-FDG PET/CT in breast cancer are monitoring the response to treatment and early detection of recurrence.22
Histopathologic features
MBCs are composed of one or more cell populations that have undergone metaplastic differentiation, meaning that the cells have transformed from glandular to non-glandular morphology. According to their behavior and histopathologic features, MBCs can be subclassified as high-grade and low-grade.13 High-grade MBC includes squamous cell carcinoma, spindle cell carcinoma, and MBC with heterologous mesenchymal differentiation, while low-grade MBC includes low-grade adenosquamous carcinoma and fibromatosis-like MBC. Mixed MBC is composed of more than one histologic subtype.
The spindle cell subtype is more likely to have an oval shape with circumscribed margins, detected both radiologically and pathologically (Figures 2, 8).1 Histologically, spindle cell carcinoma reveals atypical spindle cells arranged in various architectural patterns (Figure 8).13 Squamous cell MBCs are frequently cystic tumors in which a central cavity lined by atypical squamous cells is encircled by neoplastic cells, with various degrees of squamous differentiation and a reactive stroma (Figure 1).2,12 Squamous cell carcinoma of the breast also tends to reveal an irregularly-shaped mass (Figures 6, 7) with spiculated margins.17 MBC with heterologous mesenchymal differentiation is comprised of a mixture of mesenchymal components with carcinomatous areas that can show squamous or glandular differentiation.2 This subtype is also denominated as matrix-producing MBC. The most frequent mesenchymal (heterologous) elements seen in these tumors are cartilage and bone (Figure 7). In cases with a predominant mesenchymal component, extensive tissue sampling may be required to find the associated epithelial elements and, therefore, differentiate these tumors from sarcomas. In some cases, immunohistochemical staining can be helpful. Immunostains for epithelial markers, such as cytokeratins, may provide the proper diagnosis (Figure 7).
Figure 8.

An example of spindle cell type metaplastic breast carcinoma in a 70-year-old female patient who presented with a palpable mass in her right breast. Excisional biopsy (a) reveals a tumor with relatively well-circumscribed margins in the majority of the sections [hematoxylin and eosin (H&E), ×1.2]. In some sections (b), the margins are irregular microscopically and show infiltration to the adipose tissue (H&E, ×2.6). Photomicrograph (c) shows the tumor composed of interlacing fascicles of highly atypical spindle cells with numerous mitotic figures. The tumor cells show prominent nuclear pleomorphism, elongated to plump eosinophilic cytoplasm (H&E, ×20).
MBC prognosis and treatment
Except for fibromatous-like carcinoma and low-grade adenosquamous carcinoma, all histopathologic subtypes are aggressive, chemoresistant, and have a high tendency to metastasize. Diagnosis below the age of 40 years, skin invasion, and squamous cell component in nodal tumors are associated with a poorer outcome.5 Rakha et al.3 reported that lymph node stage, lymphovascular invasion, and histologic subtype were associated with outcome but tumor size and grade were not. Matrix-producing carcinoma is generally associated with a better prognosis compared with spindle cell and squamous cell carcinomas.3 The biological behavior of squamous cell carcinoma is similar to that of invasive ductal carcinoma with invasive growth, which explains the malignant features seen in squamous cell carcinoma.9 Tumors with benign imaging features like circumscribed margins might represent higher histologic tumor grades and aggressive malignancies associated with poor prognoses.6
Currently, there is no standard therapeutic approach for MBC. MBC tends to be resistant to conventional chemotherapy. Nevertheless, adjuvant chemotherapy remains the backbone of the treatment protocol as studies show that it improves the prognosis, particularly when administered for early-stage disease. Moreover, because these tumors are generally triple negative, hormonotherapy and targeted therapies such as trastuzumab are likely to be ineffective.
Conclusion
MBC tends to demonstrate benign imaging features, such as an oval shape and circumscribed margins on MG and US. Awareness of these overlapping findings, investigation of the clinical features, and using MRI techniques can assist in differentiating between MBC and benign tumors or other types of breast cancer. T2 high signal intensity, rim enhancement, and non-enhancing internal components on MRI would help differentiate MBC from other breast malignancies. In addition to multimodality imaging, histopathologic evaluation, including immunohistochemistry analysis, is essential for diagnosing MBC.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Günhan-Bilgen I, Memiş A, Ustün EE, Zekioglu O, Ozdemir N. Metaplastic carcinoma of the breast: clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;178(6):1421–1425. doi: 10.2214/ajr.178.6.1781421. [DOI] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board. Breast Tumours. Lyon (France): International Agency for Research on Cancer; 2019. (WHO classification of tumours series, 5th ed.; vol. 2) [Internet] https://publications.iarc.fr/581.
- 3.Rakha EA, Tan PH, Varga Z, et al. Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Br J Cancer. 2015;112(2):283–289. doi: 10.1038/bjc.2014.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velasco M, Santamaría G, Ganau S, et al. MRI of metaplastic carcinoma of the breast. AJR Am J Roentgenol. 2005;184(4):1274–1278. doi: 10.2214/ajr.184.4.01841274. [DOI] [PubMed] [Google Scholar]
- 5.McKinnon E, Xiao P. Metaplastic carcinoma of the breast. Arch Pathol Lab Med. 2015;139(6):819–822. doi: 10.5858/arpa.2013-0358-RS. [DOI] [PubMed] [Google Scholar]
- 6.Yang WT, Hennessy B, Broglio K, et al. Imaging differences in metaplastic and invasive ductal carcinomas of the breast. AJR Am J Roentgenol. 2007;189(6):1288–1293. doi: 10.2214/AJR.07.2056. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Kim SY, Huh S. Multimodality imaging findings of metaplastic breast carcinomas: a report of five cases. Ultrasound Q. 2018;34(2):88–93. doi: 10.1097/RUQ.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia Y, He C, Liu L, et al. A retrospective study of the Imaging and Pathological Features of Metaplastic Breast Carcinoma and Review of the Literature. Med Sci Monit. 2019;25:248–258. doi: 10.12659/MSM.912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y, Liu X, Zhang G, et al. Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. World J Surg Oncol. 2013;11:129. doi: 10.1186/1477-7819-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi BB, Shu KS. Metaplastic carcinoma of the breast: multimodality imaging and histopathologic assessment. Acta Radiol. 2012;53(1):5–11. doi: 10.1258/ar.2011.110341. [DOI] [PubMed] [Google Scholar]
- 11.Leddy R, Irshad A, Rumboldt T, Cluver A, Campbell A, Ackerman S. Review of metaplastic carcinoma of the breast: imaging findings and pathologic features. J Clin Imaging Sci. 2012;2:21. doi: 10.4103/2156-7514.95435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JM, Han BK, Moon WK, Choe YH, Ahn SH, Gong G. Metaplastic carcinoma of the breast: mammographic and sonographic findings. J Clin Ultrasound. 2000;28(4):179–186. doi: 10.1002/(sici)1097-0096(200005)28:4<179::aid-jcu5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.González-Martínez S, Pérez-Mies B, Carretero-Barrio I, et al. Molecular Features of Metaplastic Breast Carcinoma: An Infrequent Subtype of Triple Negative Breast Carcinoma. Cancers (Basel). 2020;12(7):1832. doi: 10.3390/cancers12071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luini A, Aguilar M, Gatti G, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101(3):349–353. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 15.Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: Analysis of 892 cases from the national cancer data base. Ann Surg Oncol. 2007;14(1):166–173. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 16.Donato H, Candelária I, Oliveira P, Gonçalo M, Caseiro-Alves F. Imaging findings of metaplastic carcinoma of the breast with pathologic correlation. J Belg Soc Radiol. 2018;102(1):46. doi: 10.5334/jbsr.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian T, Lin Q, Wu Z, et al. Metaplastic carcinoma of the breast: imaging and pathological features. Oncol Lett. 2016;12(5):3975–3980. doi: 10.3892/ol.2016.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin HJ, Kim HH, Kim SM, et al. Imaging features of metaplastic carcinoma with chondroid differentiation of the breast. AJR Am J Roentgenol. 2007;188(3):691–696. Erratum in: AJR Am J Roentgenol. 2007;188(5):1170. doi: 10.2214/AJR.05.0831. [DOI] [PubMed] [Google Scholar]
- 19.Langlands F, Cornford E, Rakha E, et al. Imaging overview of metaplastic carcinomas of the breast: a large study of 71 cases. Br J Radiol. 2016;89(1064):20140644. doi: 10.1259/bjr.20140644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YW, Lee MH, Kwon KH, et al. Magnetic resonance imaging of metaplastic carcinoma of the breast: sonographic and pathologic correlation. Acta radiol. 2004;45(1):18–22. doi: 10.1080/02841850410000773. [DOI] [PubMed] [Google Scholar]
- 21.Benveniste AP, Yang W, Benveniste MF, Mawlawi OR, Marom EM. Benign breast lesions detected by positron emission tomography-computed tomography. Eur J Radiol. 2014;83(6):919–929. doi: 10.1016/j.ejrad.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Sang KY, Cho N, Woo KM. The role of PET/CT for evaluating breast cancer. Korean J Radiol. 2007;8(5):429–437. doi: 10.3348/kjr.2007.8.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]


