Figure 5.
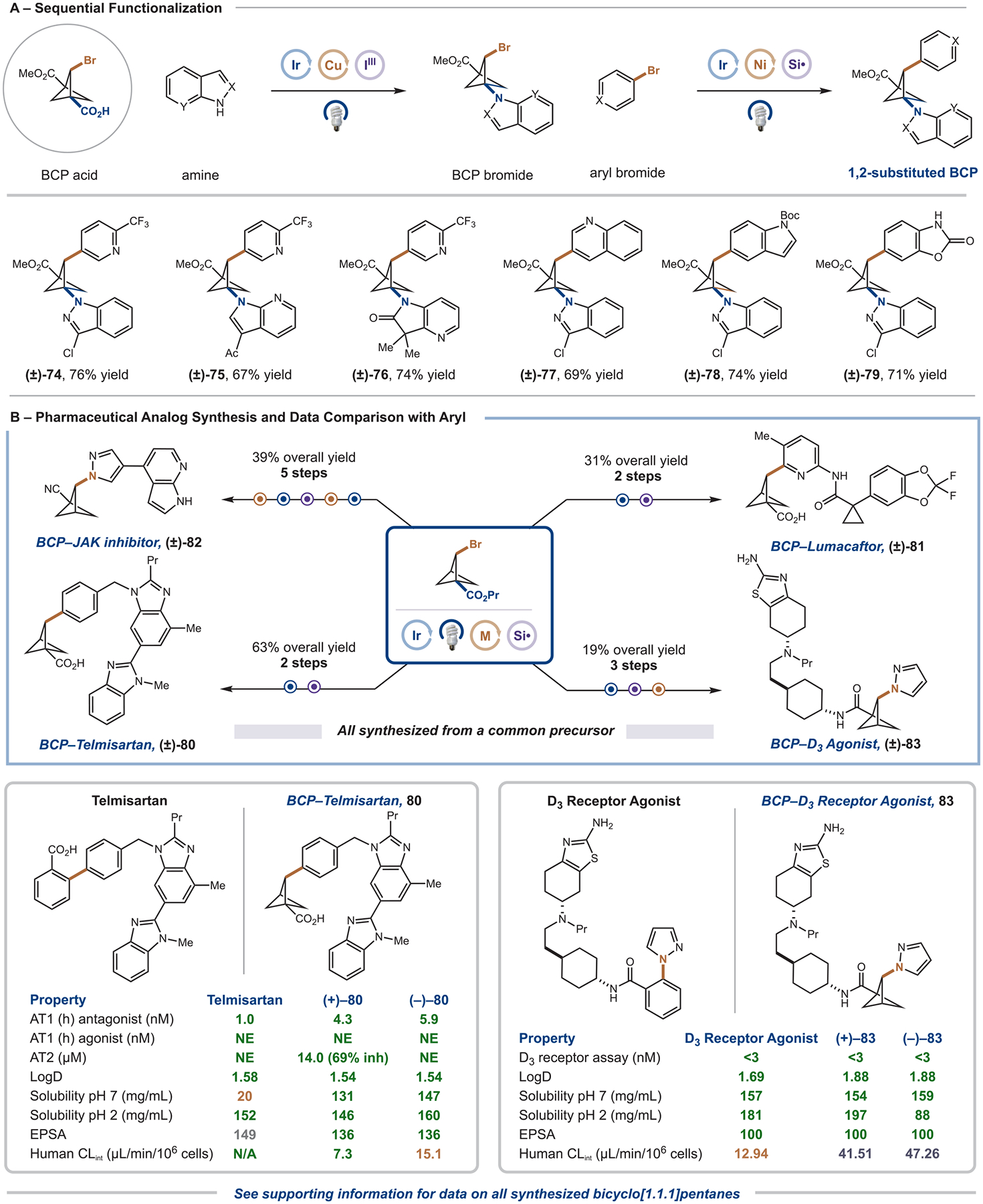
Sequential functionalization and drug analogues. (A) A 2-brominated, 1-carboxylic acid BCP can be leveraged in sequential photoredox-catalyzed decarboxylative coupling, followed by silyl-radical-mediated 2-arylation. Reported yield is for the silyl-radical-mediated 2-arylation. All yields are isolated. Standard conditions: aryl bromide (0.3 mmol, 1 equiv), BCP bromide (2 equiv), aminosilane (1.6 equiv), Ir(dF(CF3)ppy)2(dtbbpy)PF6 (2 mol %), Ni(dtbbpy)Br2 (5 mol %), Cs2CO3 (2 equiv), TFT (0.05 M), IPR 450 nm (100% intensity) for 2 h. See the Supporting Information for full experimental details. (B) These silyl-radical-mediated 2-arylation and 2-amination procedures can be applied to the rapid preparation of pharmaceutical analogues and bioactive molecules. Data is compared with the aryl compound. All yields are isolated. See the Supporting Information for full experimental details. inh, Inhibition; NE, no significant effect.
