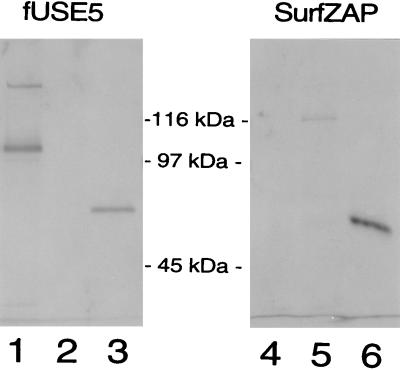
FIG. 2.
Immunoblot detection of phage-displayed Cry1Ac fusion proteins. Phages (109 TU for fUSE5 constructs and 1010 TU for SurfZAP constructs) were boiled for 4 min in 30 μl of Laemmli denaturing sample buffer and size separated by SDS–8% PAGE. The proteins were transferred to nitrocellulose and detected by a rabbit polyclonal anti-Cry1Ac antibody and then by an alkaline phosphatase-conjugated goat anti-rabbit antibody. Lane 1, 1Ac-fUSE5 phage; lane 2, WT-fUSE5 phage; lane 3, 10 ng of purified trypsin-activated HD-73 Cry1Ac; lane 4, SurfZAP-FAb phage; lane 5, SurfZAP-1Ac phage; lane 6, 10 ng of purified trypsin-activated HD-73 Cry1Ac. The blot was divided through the molecular mass markers between lanes 3 and 4, and the two sides were developed separately. The SurfZAP side of the blot was allowed to develop for twice as long as the fUSE5 side, since SurfZAP-1Ac phage produced a lower-intensity signal. The effect of the longer development time can be observed by comparing the 10-ng toxin bands on each side of the blot (lanes 3 and 6).