Fig. 2.
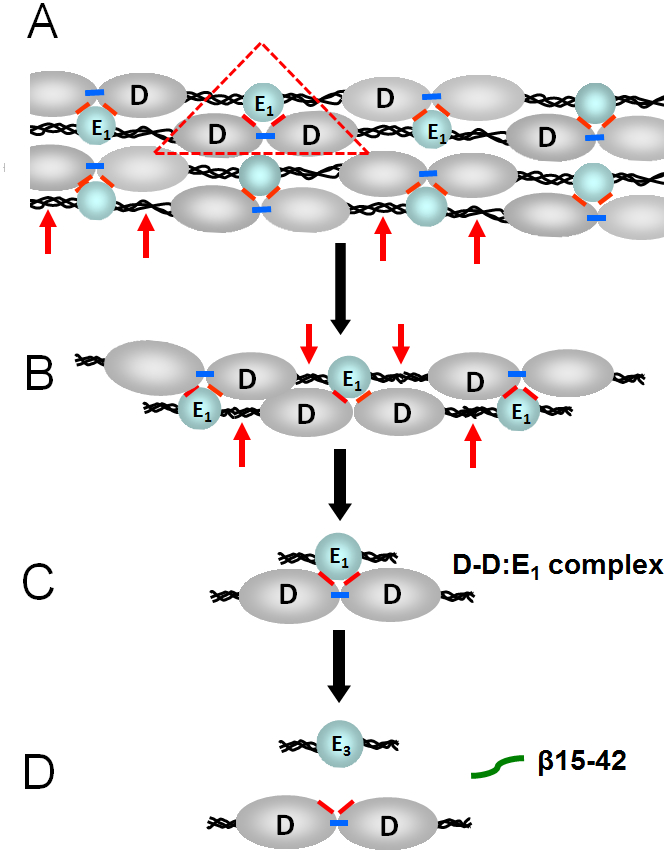
(A) Schematic representation of a fibrin polymer. D and E1 identify the D (grey color) and E (blue color) regions of individual fibrin molecules; the αC-domains are not shown for simplicity. Fibrin polymerization occurs mainly through non-covalent interactions between the complementary polymerization sites located in the D and E regions (the DD:E1 interaction shown by red triangle). Factor XIIIa crosslinks D-D regions by formation covalent bonds (shown by blue bars) between the C-terminal portions of the γ chains of these regions reinforcing fibrin polymers. Plasmin cleavage between the D and E regions of fibrin is shown by red arrows. (B and C) Cleavage of fibrin by plasmin results in high molecular mass fibrin degradation products (HMM-FDP) and the D-D:E1 complex. (D) Major final fibrin degradation products, the D-D dimer, the E3 fragment, and the β15-42 fragment.
