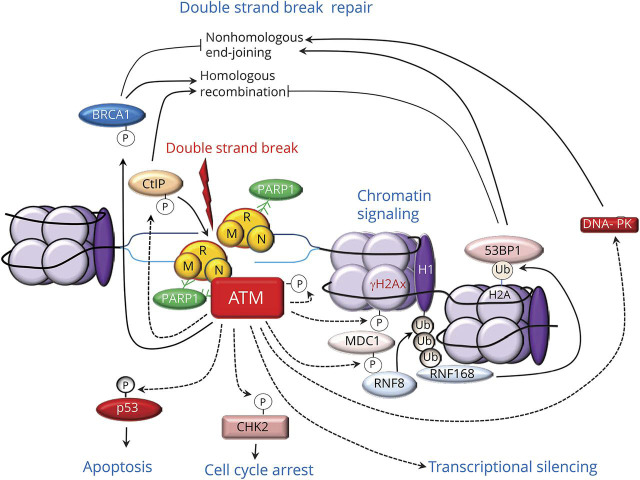
Figure 4. Multiple functions of ataxia telangiectasia mutated (ATM) protein in the DNA double-strand break response.
After binding to the site of DNA damage via interactions with poly (adenosine diphosphate [ADP] ribose) polymerase (PARP)1 and the MRN protein complex, consisting of meiotic recombination 11 (MRE11), DNA repair bridging protein 50 (RAD50), and Nijmegen breakage syndrome–1 (NBS-1), ATM phosphorylates and activates hundreds of substrate proteins, providing a mechanism for signal amplification. ATM stimulates DNA end-resection by phosphorylating C-terminal binding protein–interacting protein (CtIP), a major activator of MRE11, and breast cancer 1 (BRCA)1 protein. ATM also promotes nonhomologous end-joining by activating DNA protein kinase (DNA-PK) and facilitating the accumulation of the p53 binding protein 1 (53BP1). ATM also phosphorylates the histone variant H2AX, yielding γH2AX; this recruits the mediator of DNA damage checkpoint 1 protein (MDC1) and triggers phosphorylation-ubiquitylation (Ub) cascades that are sequentially mediated by the ring finger (RNF) 8 and RNF168 ubiquitin E3 ligases. Another target of ATM-mediated phosphorylation are the effector checkpoint kinases 2 (CHK2), which elicit cell cycle arrest; and p53, which also triggers apoptosis. ATM is recruited and activated at the site of R-loops and elicits transcriptional silencing.