Abstract
Objective
To evaluate race differences in tissue plasminogen activator (tPA) refusal among eligible patients with acute ischemic stroke (AIS) in Chicago.
Methods
Using the Get With The Guidelines–Stroke registry data from 15 primary stroke centers between January 2013 and June 2015, we performed a retrospective analysis of patients with AIS presenting to the emergency department within 4.5 hours from symptom onset. Patient or proxy refusal was captured as a reason for nonadministration of tPA to eligible patients in the registry. We assessed whether tPA refusal differed by race using logistic regression.
Results
Among 704 tPA-eligible patients with AIS, tPA was administered to 86.2% (black race, 82.5% vs nonblack race, 89.5%; p < 0.001). Fifty-three (7.5%) tPA refusals were documented. Refusal was more common in black vs nonblack patients (10.6% vs 4.8%; p = 0.004). In multivariable analysis, the following were associated with tPA refusal: black race (adjusted odds ratio [OR] 2.5, 95% confidence interval [CI] 1.3–4.6), self-pay status (adjusted OR 3.23, 95% CI 1.2–8.71), prior stroke (adjusted OR 2.11, 95% CI 1.14–3.90), age (adjusted OR 1.04, 95% CI 1.02–1.07), and NIH Stroke Scale score (adjusted OR 0.94, 95% CI 0.90–0.99).
Conclusions
Among tPA-eligible patients with AIS in Chicago, over 7% refused tPA. Refusal was more common in black patients and accounted for the apparent lower rates of tPA use in black vs nonblack patients. Further research is needed to understand barriers to consent and overcome race–ethnic disparities in tPA treatment for AIS.
Since its approval for use in patients with acute ischemic stroke (AIS) in 1996, the rate of tissue plasminogen activator (tPA) administration to eligible patients has steadily increased.1 The development and dissemination of stroke centers in the United States and hospital-based quality improvement initiatives including the American Heart Association (AHA)'s Target Stroke program have played an important role in appropriate and timely tPA use in patients with AIS.2 Yet despite these efforts, tPA utilization for AIS nationwide remains suboptimal, ranging between 3% and 7%,3,4 and with lower rates among black than nonblack patients.5
Indeed, racial and ethnic disparities have been observed in all aspects of stroke, including prehospital care, acute treatment with tPA, and poststroke outcomes.6 For example, black patients are more likely to die and be disabled after stroke and half as likely to receive tPA compared to non-Hispanic white patients.7,8 Black patients are 20% less likely to arrive by emergency medical services and are less likely to achieve door-to-needle (DTN) times <60 minutes.9,10 Racial disparities in tPA utilization persist even at primary stroke centers (PSCs).5
In a qualitative study at 2 large Chicago hospitals, we previously reported that obtaining consent may contribute to delays in DTN time and tPA refusal.11 Refusal of tPA may result from patient or caregiver uncertainty about the risks and benefits of tPA. These observations led to the development and dissemination of standardized informed consent pocket cards to Chicago hospitals participating in the regional Quality Enhancement for Speedy Thrombolysis in Stroke (QUESTS) initiative.12 Recently, more refined decision aids have been developed to assist clinicians with the informed consent process for tPA.13 However, empiric data on the prevalence of tPA refusal is sparse14 and no data exist on race–ethnic disparities in tPA refusal. Building upon these studies and our prior observations at Chicago hospitals, we hypothesized that race–ethnic disparities exist in tPA refusal among tPA-eligible patients at 15 QUESTS-participating PSCs in Chicago.
Methods
Study population
We performed a retrospective analysis of patients with AIS discharged from 15 Chicago PSCs (table e-1, links.lww.com/WNL/A104) between January 2013 and June 2015. All of the participating hospitals contribute data to the AHA Get With The Guidelines (GWTG)–Stroke registry (Quintiles, Inc., Cambridge, MA). GWTG-Stroke is a national quality improvement program focused on guideline-driven care in stroke patients.15 As part of QUESTS,12 each hospital agreed to share the data and report results in aggregate form. All data were entered by coordinators at each hospital without central adjudication, interpretation, or review. A GWTG-Stroke superuser account, managed by the AHA/American Stroke Association, was created to monitor and aggregate the regional data.
Patients with a primary diagnosis of AIS who presented to the emergency department (ED) within 4.5 hours from symptom onset were included. We excluded patients with documented medical contraindications to tPA upon arrival to the ED, patients who developed stroke symptoms after hospital arrival, and patients with incomplete documentation (e.g., missing arrival or tPA treatment times or initial NIH Stroke Scale [NIHSS] scores). Reasons for not administering tPA included medical contraindications (e.g., blood pressure control or inability to determine eligibility based upon medical history), hospital factors (delay in arrival or diagnosis), as well as patient/family refusal.16
Variable of interest
Since multiple reasons may have been documented for not administrating tPA for any one patient, we defined tPA refusal when it was the only documented reason for the purposes of analysis.
Covariates
Other relevant covariates captured in the GTWG-Stroke registry included age, sex, race/ethnicity, health insurance status, mode of arrival, arrival and admission time data, and initial NIHSS score. We simplified race–ethnicity into 3 categories: African American or black, Caucasian or white, and other (including Hispanic, Asian, Native American, undetermined, and not documented). Medical history included the presence of atrial fibrillation/flutter, coronary artery disease, diabetes mellitus, heart failure, hypertension, smoking status, prior stroke, and prior TIA.
Statistical analysis
All data analyses were performed using the Statistical Package for the Social Sciences (SPSS 24.0; IBM, Armonk, NY). Descriptive statistics are expressed as means with SD or medians with interquartile ranges (IQRs), as appropriate, for continuous variables and frequencies for categorical variables. A test for trend was performed to evaluate change in rate of refusal by quarter during the study period. To compare demographic or clinical characteristics between patients with and without tPA refusal, we performed univariable tests using χ2 tests for categorical variables and t tests or Mann-Whitney U tests for continuous variables, as appropriate. Variables were selected for the multivariable models based on univariable association (p < 0.15) with tPA refusal. To determine independent factors contributing to tPA refusal, separate multivariable logistic regression analyses were performed using a stepwise elimination approach to create a parsimoniously adjusted model with less susceptibility to overfitting and a probability of F-to-remove ≥0.1. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from the final models. The model fitness was assessed using the Hosmer-Lemeshow test. A p value <0.05 was considered significant in final models.
Standard protocol approvals, registrations, and patient consents
All participating hospitals were required to comply with local regulatory and privacy guidelines and, if necessary, to secure institutional review board approval.
Results
Of the 13,662 patient records in the regional registry during the study period, 704 (5.2%) AIS patients without documented medical contraindications to tPA were identified (figure). Sixteen patients (4 black, 12 nonblack) with documented refusals also had documented medical contraindications to tPA (e.g., recent surgery, uncontrollable blood pressure) and were not included in the study cohort.
Figure. Flowchart of study cohort assembly.
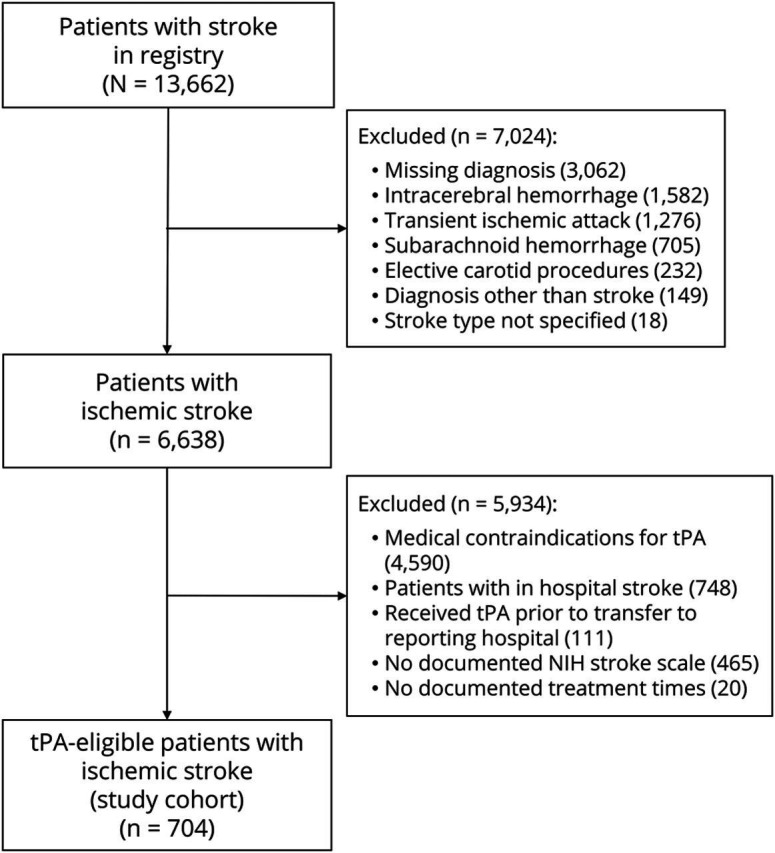
tPA = tissue plasminogen activator.
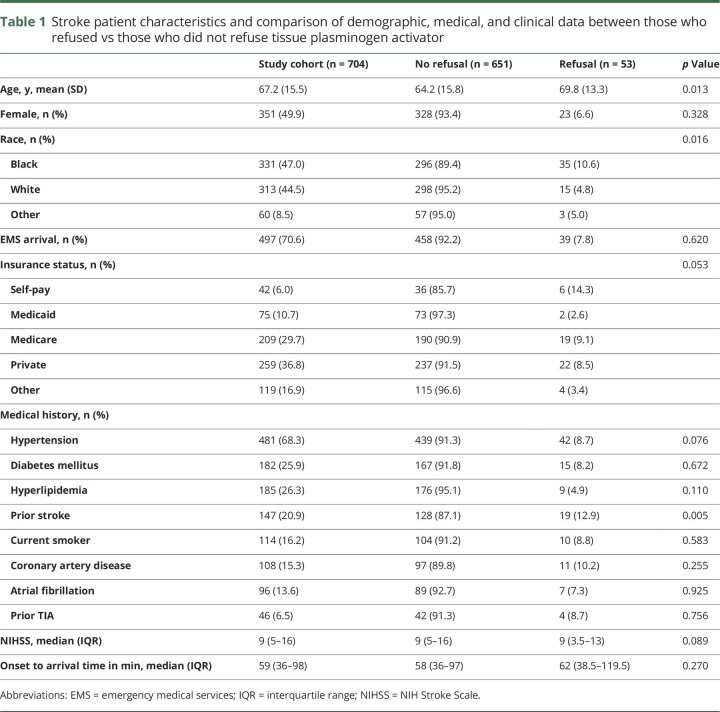
Patient characteristics of the study cohort are described in table 1. The mean age was 67.2 ± 15.5 years, 49.9% were female, and 47.0% were black. Eighty-six percent (n = 607) of eligible AIS patients received tPA (82.5% black vs 89.5% nonblack, p < 0.001). Fifty-three (7.5%) patients or their proxies refused treatment with tPA. The rate of tPA refusal did not change by quarter over the study period (p = 0.522).
Table 1.
Stroke patient characteristics and comparison of demographic, medical, and clinical data between those who refused vs those who did not refuse tissue plasminogen activator
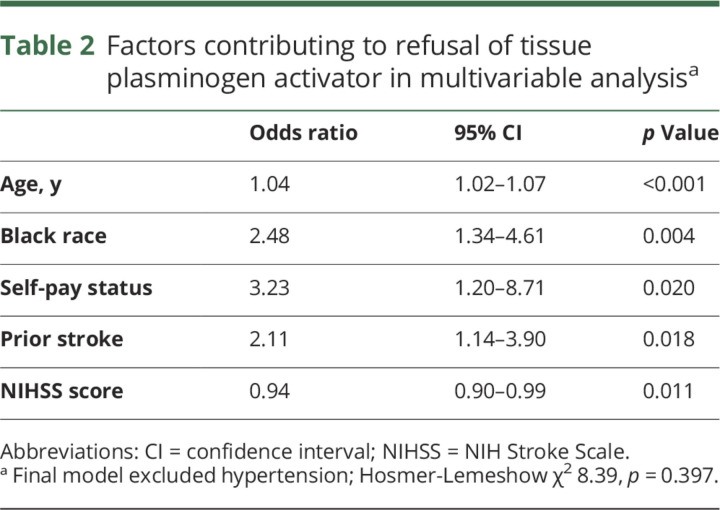
The rate of refusal was higher in black compared to nonblack patients (10.6% vs 4.8%, p = 0.004). Refusal rates were also higher in patients with a history of prior stroke (12.9% vs 6.1%, p = 0.005) and older patients (69.8 ± 13.3 vs 64.2 ± 15.8, p = 0.013). The median NIHSS was 9 (IQR 5–15) compared to 9 (3.5–13) in patients without and with tPA refusal (p = 0.089), respectively. There was no difference in the rate of refusal (p = 0.197) or race (p = 0.583) among patients presenting within 3 hours of symptom onset and patients arriving between 3 and 4.5 hours. There were no differences by sex or by arrival mode between patients who refused and those who received tPA (table 1). Based on significance in univariable analysis, we included the following variables in a multivariable model of refusal: age, race, self-pay status, prior stroke, NIHSS score, and hypertension. On multivariable analysis, black patients (or their proxies) were more likely to refuse tPA (adjusted OR 2.48, 95% CI 1.34–4.61; table 2). Other factors contributing to tPA refusal included self-pay status (adjusted OR 3.23, 95% CI 1.2–8.71), prior stroke (adjusted OR 2.11, 95% CI 1.14–3.90), age (adjusted OR 1.04, 95% CI 1.02–1.07), and NIHSS score (adjusted OR 0.94, 95% CI 0.90–0.99).
Table 2.
Factors contributing to refusal of tissue plasminogen activator in multivariable analysisa
Discussion
Among tPA-eligible AIS patients at 15 PSCs in Chicago, 7.5% did not receive tPA because of patient or proxy refusal. While the observed tPA refusal rate in Chicago is consistent with other single-center studies (4.2%–6.9%),14,17 we found that black patients were more than twice as likely to refuse tPA compared to nonblack patients. Indeed, the observed disparity in tPA administration, an absolute 7% difference in black vs nonblack patients, was nearly all attributable to higher refusal rates in black patients (5.8% absolute difference). These data suggest that cultural and community barriers to tPA consent, especially in black communities, warrant further investigation.
Current professional guidelines state that explicit, though not written, informed consent is indicated when providing tPA to eligible AIS patients.18,19 Informed consent is more frequently obtained from patient surrogates than acute stroke patients themselves because of a perceived lack in capacity20 and are often initiated by physicians in the ED. However, challenges to informed consent for tPA may stem from cognitive impairment or aphasia due to current or prior stroke, lack of availability of proxies, or misunderstanding potential risk–benefit ratios.
Providing informed consent requires effective communication among health care providers, patients, and their surrogates. Our data also imply a need to tailor the informed consent process to individual patient and cultural characterisitics.21 Some patients may not be aware that tPA is an approved medical treatment and not an investigational drug, that treatment benefits are time-dependent, and that overall benefits outweigh risks.22 Although socioeconomic differences between black and white patients may contribute to health care disparities, they only explain a portion of the disparities.23,24 Social determinants of health, such as residential environments,25 social support,26 and knowledge of available therapies,27 affect outcomes after stroke. Disparities may also be due to varying levels of health literacy among black vs nonblack patients. Though some have noted that health literacy for stroke treatments is poor in general,28 health numeracy is suboptimal especially in elderly patients, minorities, and those with lower education and socioeconomic status.29 Racial and ethnic disparities in health communication, heavily influenced by health literacy and numeracy, are known to exist in clinical situations such as acute stroke.30,31 Another possible factor, institutional mistrust in health care, is also higher in black compared to non-Hispanic white patients and may contribute to tPA refusal.32
To satisfy the time constraints of administering tPA in patients with AIS, strategies to improve patient or proxy understanding of the indication, risks, and benefits of tPA in a timely manner, such as use of structured oral presentations and visual aids,33 have been described.34 Recently, more detailed tools have been developed to aid AIS patients and their proxies in tPA decision-making.13,35 One decision aid, for example, uses a mobile phone application to provide probabilities of independence and death following stroke treatment based on patient characteristics (e.g., age, sex, NIHSS).35 Another, the Rapid Evaluation for Stroke Outcomes using Lytics in Vascular Event (RESOLVE) tool, uses 3 printed pages of patient-facing materials that include a description of ischemic stroke along with 2 pages of population and individual-level data regarding risks and benefits of tPA.13 Future strategies to tailor informed consent conversations will need to achieve satisfactory information exchange in a culturally appropriate manner while avoiding unnecessary treatment delays or refusals, which could result in poorer outcomes. Some have advocated a different approach, framing the discussion as informed refusal rather than informed consent.36 Regardless of the framing, the discussion must ensure key elements (e.g., assessing capacity or evaluating comprehension) are addressed within the time constraints of acute stroke care and tailored to the needs of black and other minority populations. Community participatory efforts such as the ongoing Patient-Centered Outcomes Research Initiative–funded Community Engagement for Early Recognition and Immediate Action in Stroke (CEERIAS) in Chicago, which aims to adapt public education in acute stroke recognition and action to cultural and neighborhood factors, could provide the methodologic framework to tailor informed consent discussions based on community and stakeholder input.
Besides race differences in tPA refusal, we confirm a prior observation that tPA refusal was inversely related to stroke severity.14 We also identified self-pay status and history of prior stroke as independent factors related to tPA refusal. Payer status and socioeconomic status are highly correlated, and lower socioeconomic status has been associated with worse outcomes and mortality from stroke.37 There has not been a demonstrated link, though, between socioeconomic status and tPA rates in AIS.38,39 As with mild stroke patients, prior stroke survivors may refuse tPA due to misperceived risks of disability. In addition, we cannot exclude the possibility that prior stroke patients with language or cognitive deficits may have been unable to clearly provide informed consent for tPA. Clearly, further research with specific attention to the informed consent process needs to be conducted to confirm our findings and to establish causal relationships.
The study has several limitations. First, the data represent practice at 15 PSCs in Chicago and therefore may not be generalizable to other regions or practice settings. Second, as a retrospective analysis of data quality improvement initiative, the study is limited by voluntary clinician and hospital reporting of tPA refusal. While consecutive patient capture and data entry are strongly recommended by the Chicago regional stroke advisory committee and were incentivized by QUESTS during the study period, we cannot exclude the possibility of missing cases and sampling occurring at some participating hospitals. Third, while pocket cards to standardize tPA consent discussions were provided to all 15 PSCs, we cannot be certain these were used in each tPA-eligible patient or know specifically what led to tPA refusal. Fourth, the accuracy of the registry data was not independently validated, especially for documentation of tPA refusal, though a prior audit of the GWTG-Stroke registry demonstrated excellent data quality overall.40 Finally, we excluded 16 patients who refused tPA but also had medical contraindications since it is impossible to know whether tPA refusal was the primary reason for not administering tPA or whether refusal was influenced by those other contraindications. We also did not consider initial refusal with subsequent delayed receipt of tPA. Thus, our results may underestimate the rate of any tPA refusal and its effect on treatment rates and times.
Among tPA-eligible AIS patients in Chicago, 7.5% refused tPA, with refusal occurring more frequently in black than nonblack patients. Refusal nearly completely accounted for the race–ethnic disparity in tPA treatment rates between black and nonblack patients. Besides quantifying the prevalence of tPA refusal, our data affirm the need and potential effect of a culturally tailored shared decision-making aid for informed consent.
Glossary
- AHA
American Heart Association
- AIS
acute ischemic stroke
- CI
confidence interval
- DTN
door-to-needle
- ED
emergency department
- GWTG
Get With The Guidelines
- IQR
interquartile range
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- PSC
primary stroke center
- QUESTS
Quality Enhancement for Speedy Thrombolysis in Stroke
- tPA
tissue plasminogen activator
Footnotes
Editorial, page 203
Podcast: NPub.org/jmm8ur
Author contributions
Scott J. Mendelson: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. Neelum T. Aggarwal: critical revision of manuscript for intellectual content. Christopher Richards: critical revision of manuscript for intellectual content. Kathleen O'Neill: acquisition of data. Jane L. Holl: analysis and interpretation of data, critical revision of manuscript for intellectual content. Shyam Prabhakaran: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content, study supervision.
Study funding
No targeted funding reported.
Disclosure
S.J. Mendelson reports no disclosures relevant to the manuscript. N.T. Aggarwal is Co-PI for the PCORI-funded CEERIAS study. C. Richards, K. O'Neill, and J.L. Holl report no disclosures relevant to the manuscript. S. Prabhakaran is PI for the PCORI-funded CEERIAS study and lead investigator for the AHA-funded QUESTS initiative. Go to Neurology.org/N for full disclosures.
References
- 1.Fonarow GC, Reeves MJ, Smith EE, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get With the Guidelines–Stroke. Circ Cardiovasc Qual Outcomes 2010;3:291–302. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014;311:1632–1640. [DOI] [PubMed] [Google Scholar]
- 3.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke 2011;42:1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines–Stroke hospitals. Circ Cardiovasc Qual Outcomes 2013;6:543–549. [DOI] [PubMed] [Google Scholar]
- 5.Aparicio HJ, Carr BG, Kasner SE, et al. Racial disparities in intravenous recombinant tissue plasminogen activator use persist at primary stroke centers. J Am Heart Assoc 2015;4:e001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2091–2116. [DOI] [PubMed] [Google Scholar]
- 7.Johnston SC, Fung LH, Gillum LA, et al. Utilization of intravenous tissue-type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity. Stroke 2001;32:1061–1068. [DOI] [PubMed] [Google Scholar]
- 8.Hsia AW, Edwards DF, Morgenstern LB, et al. Racial disparities in tissue plasminogen activator treatment rate for stroke: a population-based study. Stroke 2011;42:2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation 2011;123:750–758. [DOI] [PubMed] [Google Scholar]
- 10.Ekundayo OJ, Saver JL, Fonarow GC, et al. Patterns of emergency medical services use and its association with timely stroke treatment: findings from get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes 2013;6:262–269. [DOI] [PubMed] [Google Scholar]
- 11.Prabhakaran S, Khorzad R, Brown A, Nannicelli AP, Khare R, Holl JL. Academic-community hospital comparison of vulnerabilities in door-to-needle process for acute ischemic stroke. Circ Cardiovasc Qual Outcomes 2015;8:S148–S154. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakaran S, Lee J, O'Neill K. Regional learning collaboratives produce rapid and sustainable improvements in stroke thrombolysis times. Circ Cardiovasc Qual Outcomes 2016;9:585–592. [DOI] [PubMed] [Google Scholar]
- 13.Decker C, Chhatriwalla E, Gialde E, et al. Patient-centered decision support in acute ischemic stroke: qualitative study of patients' and providers' perspectives. Circ Cardiovasc Qual Outcomes 2015;8:S109–S116. [DOI] [PubMed] [Google Scholar]
- 14.Vahidy FS, Rahbar MH, Lal AP, Grotta JC, Savitz SI. Patient refusal of thrombolytic therapy for suspected acute ischemic stroke. Int J Stroke 2015;10:882–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwamm LH, Fonarow GC, Reeves MJ, et al. Get With the Guidelines–Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation 2009;119:107–115. [DOI] [PubMed] [Google Scholar]
- 16.QuintilesIMS and The American Heart Association I. Stroke patient management tool coding key [online]. Available at: osstatic.outcome.com/online_doc_qi/StrokePMT/crf/StrokeCodingKey.pdf. Accessed June 30, 2017.
- 17.Huang P, Khor GT, Chen CH, Lin RT, Liu CK. Eligibility and rate of treatment for recombinant tissue plasminogen activator in acute ischemic stroke using different criteria. Acad Emerg Med 2011;18:273–278. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Neurology policy on consent issues for the administration of IV tPA [online]. Available at: aan.com/uploadedFiles/Website_Library_Assets/Documents/6.Public_Policy/1.Stay_Informed/2.Position_Statements/3.PDFs_of_all_Position_Statements/IV.pdf. Accessed July 21, 2016.
- 19.Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum JR, Bravata DM, Concato J, Brass LM, Kim N, Fried TR. Informed consent for thrombolytic therapy for patients with acute ischemic stroke treated in routine clinical practice. Stroke 2004;35:e353–e355. [DOI] [PubMed] [Google Scholar]
- 21.Gong J, Zhang Y, Feng J, et al. How best to obtain consent to thrombolysis: individualized decision-making. Neurology 2016;86:1045–1052. [DOI] [PubMed] [Google Scholar]
- 22.Kleindorfer D, Khoury J, Broderick JP, et al. Temporal trends in public awareness of stroke: warning signs, risk factors, and treatment. Stroke 2009;40:2502–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the Reasons for Geographic and Racial Differences in Stroke study. Stroke 2006;37:1171–1178. [DOI] [PubMed] [Google Scholar]
- 24.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 25.Brown AF, Liang LJ, Vassar SD, et al. Neighborhood disadvantage and ischemic stroke: the Cardiovascular Health Study (CHS). Stroke 2011;42:3363–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawachi I, Colditz GA, Ascherio A, et al. A prospective study of social networks in relation to total mortality and cardiovascular disease in men in the USA. J Epidemiol Community Health 1996;50:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutfiyya MN, Lipsky MS, Bales RW, Cha I, McGrath C. Disparities in knowledge of heart attack and stroke symptoms among adult men: an analysis of behavioral risk factor surveillance survey data. J Natl Med Assoc 2008;100:1116–1124. [DOI] [PubMed] [Google Scholar]
- 28.Fang MC, Panguluri P, Machtinger EL, Schillinger D. Language, literacy, and characterization of stroke among patients taking warfarin for stroke prevention: implications for health communication. Patient Educ Couns 2009;75:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SG, Wolf MS, von Wagner C. Socioeconomic status, statistical confidence, and patient-provider communication: an analysis of the Health Information National Trends Survey (HINTS 2007). J Health Commun 2010;15(suppl 3):169–185. [DOI] [PubMed] [Google Scholar]
- 30.Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians' implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health 2012;102:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flickinger TE, Saha S, Roter D, et al. Respecting patients is associated with more patient-centered communication behaviors in clinical encounters. Patient Educ Couns 2016;99:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwei RJ, Kadunc K, Nguyen AL, Jacobs EA. Impact of sociodemographic factors and previous interactions with the health care system on institutional trust in three racial/ethnic groups. Patient Educ Couns 2014;96:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gadhia J, Starkman S, Ovbiagele B, Ali L, Liebeskind D, Saver JL. Assessment and improvement of figures to visually convey benefit and risk of stroke thrombolysis. Stroke 2010;41:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi HY, Kim EH, Yoo J, et al. Decision-making support using a standardized script and visual decision aid to reduce door-to-needle time in stroke. J Stroke 2016;18:239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMeekin P, Flynn D, Ford GA, Rodgers H, Gray J, Thompson RG. Development of a decision analytic model to support decision making and risk communication about thrombolytic treatment. BMC Med Inform Decis Mak 2015;15:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwamm LH. Acute stroke: shifting from informed consent to informed refusal of intravenous tissue-type plasminogen activator. Circ Cardiovasc Qual Outcomes 2015;8:S69–S72. [DOI] [PubMed] [Google Scholar]
- 37.Addo J, Ayerbe L, Mohan KM, et al. Socioeconomic status and stroke: an updated review. Stroke 2012;43:1186–1191. [DOI] [PubMed] [Google Scholar]
- 38.Kerr GD, Higgins P, Walters M, et al. Socioeconomic status and transient ischaemic attack/stroke: a prospective observational study. Cerebrovasc Dis 2011;31:130–137. [DOI] [PubMed] [Google Scholar]
- 39.Arrich J, Mullner M, Lalouschek W, Greisenegger S, Crevenna R, Herkner H. Influence of socioeconomic status and gender on stroke treatment and diagnostics. Stroke 2008;39:2066–2072. [DOI] [PubMed] [Google Scholar]
- 40.Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association get with the guidelines-stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J 2012;163:392–398, 398.e1. [DOI] [PubMed] [Google Scholar]