Abstract
Cannabis is the most widely used recreational drug in the United States and regular use has been linked to deficits in attention and memory. However, the effects of regular use on motor control are less understood, with some studies showing deficits and others indicating normal performance. Eighteen users and 23 nonusers performed a motor sequencing task during high‐density magnetoencephalography (MEG). The MEG data was transformed into the time‐frequency domain and beta responses (16–24 Hz) during motor planning and execution phases were imaged separately using a beamformer approach. Whole‐brain maps were examined for group (cannabis user/nonuser) and time window (planning/execution) effects. As expected, there were no group differences in task performance (e.g., reaction time, accuracy, etc.). Regular cannabis users exhibited stronger beta oscillations in the contralateral primary motor cortex compared to nonusers during the execution phase of the motor sequences, but not during the motor planning phase. Similar group‐by‐time window interactions were observed in the left superior parietal, right inferior frontal cortices, right posterior insular cortex, and the bilateral motor cortex. We observed differences in the neural dynamics serving motor control in regular cannabis users compared to nonusers, suggesting regular users may employ compensatory processing in both primary motor and higher‐order motor cortices to maintain adequate task performance. Future studies will need to examine more complex motor control tasks to ascertain whether this putative compensatory activity eventually becomes exhausted and behavioral differences emerge.
Keywords: beta, gamma, marijuana, movement, motor sequencing, oscillations
Recreational cannabis use is becoming increasingly more common, but the long‐term impact of regular use on the brain is poorly understood. Herein, we investigated how regular cannabis use affects the brain circuitry and dynamics serving motor control in adults. We found that regular cannabis use alters the dynamics serving motor control in primary and higher‐order motor cortices.

1. INTRODUCTION
Cannabis is the most widely used recreational drug in the United States, with an estimated 49.6 million individuals 12 years of age and older reporting use in 2020, which is nearly 18% of the population (Substance Abuse and Mental Health Services Administration, 2020). Among young adults between the ages of 19–30 years, usage has increased dramatically over the last 10 years, from 29.4% in 2011 to an all‐time high of 42.6% of young adults reporting usage in 2021. Regular consumption has also nearly doubled, from 5.7% of young adults reporting daily usage in 2011 to 10.8% in 2021. Adults between the ages of 35–50 have displayed similar increasing patterns of cannabis consumption, with 12.6% reported using cannabis in 2011 compared to 24.9% in 2021 (Patrick et al., 2022). Given these sharp upward trends in the use of cannabis, it is becoming increasingly important to understand its effects on the brain.
Acute cannabis intoxication is known to cause impairments across a wide array of cognitive processes. Attention and memory are among the most well‐studied neurocognitive domains with respect to cannabis use, and intoxication has long been known to negatively affect these domains in a dose‐dependent manner (Anderson et al., 2010; D'Souza, Braley, et al., 2008; D'Souza, Ranganathan, et al., 2008; Ferraro, 1980). On the other hand, findings from studies examining cognitive domains such as decision making, psychomotor control, and inhibitory control tend to be more equivocal (Crane et al., 2013; Kroon et al., 2021). For example, some studies investigating inhibitory control have found that in tasks requiring participants to inhibit movement that is already ongoing, such as in the stop‐signal task (Logan et al., 1984), cannabis intoxication causes a dose‐dependent reduction in participants' ability to stop (Kroon et al., 2021; McDonald et al., 2003; Metrik et al., 2012). By contrast, other studies have found that cannabis may not impair inhibition in the context of inhibiting before a response is initiated (e.g., in go/no‐go tasks; Crane et al., 2013; Donders, 1969; Kroon et al., 2021; McDonald et al., 2003).
Interestingly, the cognitive consequences of regular cannabis use, the definition of which can vary widely from study to study, is less understood with individual studies frequently reporting conflicting results. Such inconsistent findings may reflect the inherent heterogeneity of real‐world cannabis use (i.e., type, dose, frequency, polysubstance use, etc.) or the multitude of different methods and tests used in this literature. Meta‐analyses of the neurocognitive effects of long‐term cannabis use have generally come to the consensus that cognitive deficits are detectable and persist after acute use (Duperrouzel et al., 2020; Schreiner & Dunn, 2012). These meta‐analyses have demonstrated significant effects across several neurocognitive domains, with learning, memory, attention, and executive function showing the strongest residual deficits in regular cannabis users. Although less robust, motor and processing speed domains have also been frequently identified as showing residual effects of cannabis use (Duperrouzel et al., 2020). However, the literature is considerably sparser with respect to the effects of regular use on psychomotor function, though some studies have found impairments in cerebellum‐dependent functions, such as motor adaptation and learning in chronic users (Bosker et al., 2013; Herreros et al., 2019). Additionally, one functional magnetic resonance imaging (FMRI) study found decreased BOLD activity in the primary motor, supplementary motor, and anterior cingulate cortices in chronic users during a finger tapping task (Pillay et al., 2008). Regular use may also lead to addiction, which is thought to be at least partially attributable to deficits in brain regions that serve behavioral inhibition (Everitt & Robbins, 2005; Goldstein & Volkow, 2002).
While studies of regular cannabis use have shown deficits in the motor domain, far less is known about the underlying brain mechanisms. The neurophysiology of motor control has been extensively studied in healthy adults and children. These studies have shown that prior to and during movement, there is a robust event‐related desynchronization (ERD) in the beta frequency band (16–24 Hz; β‐ERD) within the motor cortex contralateral to the movement (and to a lesser magnitude, the ipsilateral motor cortex), which is followed by a post‐movement beta rebound (PMBR) that peaks after movement termination and is located slightly posterior to the β‐ERD (Heinrichs‐Graham et al., 2016, 2018; Heinrichs‐Graham, Kurz, et al., 2014; Jurkiewicz et al., 2006; Wiesman et al., 2020; Wilson et al., 2010). The amplitude of the β‐ERD changes as a function of difficulty, such that as task complexity increases, the magnitude also increases (Heinrichs‐Graham & Wilson, 2015; Pfurtscheller & Lopes da Silva, 1999). The β‐ERD has been repeatedly linked to motor planning and execution, whereas the PMBR is thought to reflect active inhibition of the motor cortex following movement termination and/or sensory feedback to the motor cortices (Arpin et al., 2017; Embury et al., 2019; Heinrichs‐Graham, Kurz, et al., 2017; Hoffman et al., 2019; Ng et al., 2011; Pfurtscheller & Lopes da Silva, 1999; Tzagarakis et al., 2010). Finally, there is a movement‐related gamma synchronization (70–90 Hz) that coincides with movement onset, is highly transient, and typically centered on the primary motor cortex following mototopic organization (Spooner, Arif, et al., 2021; Spooner & Wilson, 2022, 2023; Wiesman et al., 2020). This response is thought to reflect the motor execution signal but is not observed in all tasks (Heinrichs‐Graham et al., 2020; Wiesman et al., 2020, 2021).
With the dramatic increase in cannabis use over the last decade, understanding the impact on human brain function has never been more important. Herein, we focus on identifying whether regular cannabis use affects motor planning and execution related neural oscillations in otherwise healthy adults. To this end, we used magnetoencephalographic (MEG) imaging and time series analysis to examine the effects of regular cannabis use on the neural oscillatory dynamics serving the planning and execution of motor sequences in adults who regularly use cannabis and a demographically matched nonuser control group. We hypothesized that cannabis users would exhibit stronger β‐ERD responses during the planning and/or execution of motor sequences compared to controls, and that these stronger responses would reflect compensatory activity that enables cannabis users to perform at normal levels on our relatively simple sequencing task.
2. METHODS
2.1. Participants
Forty‐five participants between the ages of 20–59 years were recruited for this study, including 21 regular cannabis users (mean age: 40.48 [SD: 9.80], range: 25–57 years) and 24 nonuser controls (mean age: 41.21 [SD: 11.22], range: 20–59 years). Potential participants were recruited from the Omaha, Nebraska metropolitan area through flyers, social media advertisements, and from participation in other studies in our laboratory. Participants were matched on age, sex, race, and Alcohol use disorder identification test (AUDIT) scores. Cannabis users were required to have used cannabis at least three times per week for three or more years, and nonusers were required to have never used cannabis or other illicit substances other than limited past experimental use, nor have used any illicit substances within the past 3 months. Substance use patterns were evaluated by interview using the NIDA Quick Screen, NIDA Modified Alcohol, Smoking and Substance Involvement Screening Test (NM‐ASSIST), and the Structured Clinical Interview for DSM‐5 Research Version‐ Module E: Substance Use Disorders (SCID‐5‐RV). Self‐administered surveys were also used, including the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory; Cannabis Use Disorder Identification Test (CUDIT); and AUDIT. Additionally, participants underwent a urinalysis to identify the use of illicit drugs. Exclusion criteria for the study included any diagnosed neurological or psychiatric disorder, any medical illness associated with CNS dysfunction, history of head trauma, current substance use disorder other than cannabis, the presence of metallic implants that could impede MEG or magnetic resonance imaging (MRI) data acquisition, and pregnancy. All study procedures were approved by the local Institutional Review Board and all participants provided written informed consent prior to participating.
2.2. Experimental paradigm
We used a motor paradigm designed by Heinrichs‐Graham and Wilson (2016) in which participants were instructed to place their right hand on a button pad and view a fixation cross for 3750 ms during MEG recording. Three numbers, each corresponding to a digit on the hand (i.e., “1” for index, “2” for middle, etc.), were shown on the screen for 500 ms. The numbers on the screen changed color, indicating the cue to move, and participants then had 2250 ms to tap the sequence with the corresponding fingers. After the 2250 ms, the numbers disappeared, leaving only the fixation cross on the screen. Following of period of 3750 ms, the next three numbers appeared (Figure 1a). The experiment consisted of 160 pseudo‐randomized trials resulting in about 16 min of total recording time.
FIGURE 1.
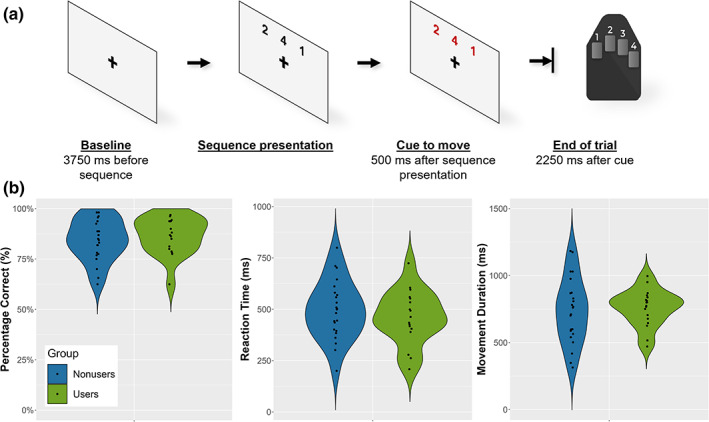
Task paradigm and behavioral performance. (a) Participants fixated on a crosshair for 3750 ms. After this period, a sequence of three numbers, each corresponding to a digit on the right hand, was displayed on the screen for 500 ms. Numbers then changed color, indicating the cue to move, with the sequence disappearing after 2250 ms (i.e., the participant had 2250 ms to complete the sequence). (b) Behavioral results for both groups. Percentage correct (accuracy) is shown on the left, reaction time (time between cue to move and movement onset) in the middle, and movement duration (time to complete the sequence) on the right. There were no main effects of group for accuracy (p = .487), reaction time (p = .631), or movement duration (p = .707).
2.3. MEG and MRI data acquisition
Functional MEG data were collected using an Elekta/MEGIN MEG system (Helsinki, Finland) equipped with 306 sensors (204 planar gradiometers, 102 magnetometers) using a 1 kHz sampling rate and an acquisition bandwidth of 0.1–330 Hz in a one‐layer magnetically shielded room with active shielding engaged. Prior to MEG acquisition, four coils were attached to the participant's head and localized along with fiducial and scalp surface points using a three‐dimensional digitizer (FASTRAK 3SF0002, Polhemus Navigator Sciences, Colchester, Vermont). Once the participants were positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils, thus inducing a measurable magnetic field and thereby allowing each coil to be localized in reference to the MEG sensor array throughout the recording session. Structural T1‐weighted images were collected using a Siemens Prisma 3.0T scanner with a 64‐channel head coil (TR: 2.3 s; TE: 2.98 ms; field of view: 256 mm; slice thickness: 1 mm with no gap; voxel size: 1 mm3). Each participant's MRI data underwent AC/PC alignment, inhomogeneity correction, segmentation, surface reconstruction, and transformation into standardized space.
2.4. Time‐frequency transformation and statistics
MEG data were subjected to environmental noise reduction and corrected for head motion using the signal space separation method with a temporal extension (Taulu & Simola, 2006). Eye blinks and cardiac artifacts were removed from the data using signal space projection (SSP), which was accounted for during source reconstruction (Uusitalo & Ilmoniemi, 1997). The time series was then divided into 6400 ms epochs, including a baseline window from −2250 to −1750 ms, with 0 ms being defined as movement onset. Epochs containing artifacts were then rejected based on a fixed threshold method that was set per participant and supplemented with visual inspection. Briefly, in MEG, the raw signal amplitude is strongly affected by the distance between the brain and the MEG sensor array, as the magnetic field strength falls off sharply as the distance from the current source (i.e., brain) increases. To account for this source of variance across participants, as well as other sources of variance, we used an individualized threshold based on the signal distribution for both amplitude and gradient to reject artifacts. There were no differences in the number of accepted trials by group (t 39 = −.728, p = .607).
Artifact‐free epochs were transformed into the time‐frequency domain using complex demodulation (Kovach & Gander, 2016; Papp & Ktonas, 1977). The resulting spectral power estimations per sensor were averaged across trials, generating time‐frequency plots of mean spectral density. The sensor‐level data were then normalized per time‐frequency bin using the respective bin's baseline power, which was calculated by averaging the power during the −2250 to −1750 ms baseline period. The specific time‐frequency bins used for source reconstruction were determined using a mass univariate approach based on the general linear model. To reduce the risk of false‐positive results while maintaining reasonable sensitivity, a two‐stage procedure was followed to control for Type‐1 error. In the first stage, two‐tailed paired‐sample t‐tests against baseline were conducted on each data point, and the output spectrogram of t‐values was thresholded at p < .05 to define time‐frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time‐frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the threshold (p < .05), and a cluster value was derived by summing the t‐values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters (from stage 1) were tested directly using this distribution (Ernst, 2004; Maris & Oostenveld, 2007) For each comparison, 1000 permutations were computed. Based on these analyses, the time‐frequency windows that contained significant oscillatory events across all participants were subjected to the beamforming analysis. For further details on our data processing pipeline, see Wiesman and Wilson (2020).
2.5. MEG source imaging and virtual sensor analyses
Oscillatory neural responses were imaged using the dynamic imaging of coherent sources beamformer (Gross et al., 2001; Van Veen et al., 1997), which applies spatial filters in the time‐frequency domain to calculate voxel‐wise source power for the entire brain volume. The single images were derived from the cross‐spectral densities of all combinations of MEG gradiometers averaged over the time‐frequency range of interest and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, we computed noise‐normalized source power for each voxel per participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth (Hillebrand et al., 2005) at a resolution of 4.0 × 4.0 × 4.0 mm. Such images are typically referred to as pseudo‐t maps, with units (pseudo‐t) that reflect noise‐normalized power differences (i.e., active versus passive) per voxel. MEG preprocessing and imaging used the Brain Electrical Source Analysis (BESA V7) software. To assess the neuroanatomical basis of the significant oscillatory responses identified through the sensor‐level analysis, grand‐average whole‐brain maps were computed.
To examine the temporal dynamics of neural activity, virtual sensor data were extracted from the peak voxel in the grand‐averaged image by applying the sensor weighting matrix derived from the forward solution to the preprocessed signal vector. Following time‐frequency domain transformation of these virtual sensor data, the envelope of spectral power was computed for the frequency range used in the beamformer analysis (i.e., 16–24 Hz, see below), yielding a time series of beta band amplitude for the entire epoch. These beta band time series were then averaged over time windows of interest (i.e., planning and execution) for group‐wise comparisons.
2.6. Statistical analyses and software
All statistical analyses, except whole‐brain models, were performed using IBM SPSS Statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Data plots were created using ggplot2 (Wickham, 2016). Two‐sample t‐tests were used to examine group differences in reaction time, movement duration, and accuracy. Analysis of the virtual sensor time series used a mixed‐model Analysis of variance (ANOVA) of the relative amplitude time series prior to and following movement execution (model = group‐by‐window). Whole‐brain statistics for the oscillatory maps were performed using the MATLAB‐based Multivariate and Repeated Measures toolbox (McFarquhar et al., 2016). For whole‐brain statistics, we used a mixed‐model ANOVA, with group (cannabis users versus nonusers) as a between‐subjects factor and time window (planning versus execution) as a within‐subjects factor. Main effects (i.e., group and window) and interaction effects were examined using a threshold of p < .001, and adjusted for multiple comparisons using a spatial extent threshold (i.e., cluster restriction; k = 8 contiguous voxels) based on the theory of Gaussian random fields (Poline et al., 1995; Worsley et al., 1996, 1999). Finally, to reduce the impact of outliers on statistical analyses, participants with values 2.5 SDs above or below their respective group mean were excluded.
3. RESULTS
Four participants were excluded from all analyses due to poor performance and/or noisy MEG data. The remaining 41 participants were between the ages of 20 and 59 years and included 18 regular cannabis users (mean age: 41.56 [SD: 9.77) years] and 23 nonuser controls (mean age: 40.78 [SD: 11.27] years). Both groups scored within the normal range on the Beck Depression Inventory. Participants were matched on age, sex, race, and AUDIT scores (Table 1).
TABLE 1.
Group‐wise demographic, substance use, and behavioral data.
| Cannabis users (n = 18) | Nonusers (n = 23) | p | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 41.56 (9.77) | 40.78 (11.27) | .819 |
| Sex (M/F) | 9/9 | 16/7 | .202 |
| Race (White/Black/other) | 15/1/2 | 17/3/3 | .674 |
| Substance use | |||
| CUDIT, median (range) | 11 (4–22) | – | – |
| AUDIT, median (range) | 4 (0–14) | 2 (0–9) | .159 |
| Cannabis SIS, median (range) a | 9 (4–17) | – | – |
| Frequency of use, count (2–4× per week/5–7× per week/more than daily) a | 2/6/9 | – | – |
| Last use, count (day before/day of scan) a | 14/3 | – | – |
| Behavioral performance | |||
| Accuracy, % (range) | 86.67 (46.25–100) | 84.51 (47.50–98.75) | .487 |
| Mean reaction time, ms (SD) | 490.79 (135.13) | 512.03 (146.71) | .414 |
| Mean movement duration, ms (SD) | 771.43 (154.64) | 747.13 (250.57) | .734 |
Abbreviations: AUDIT, Alcohol Use Disorder Identification Test; CUDIT, Cannabis Use Disorder Identification Test; Cannabis SIS, Cannabis Substance Involvement Score from NIDA Modified Alcohol, Smoking, and Substance Involvement Screening Test.
One participant declined to respond.
3.1. Behavioral performance
To assess task performance, two‐sample t‐tests were conducted on the accuracy, reaction time, and movement duration data (i.e., the time from the first button press to the last in a single trial). The results indicated that the two groups did not statistically differ in accuracy (users: M = 86.67% [SD = 0.091]; nonusers: M = 84.51 [SD = 0.103]; p = .487), reaction time (users: M = 460.150 [SD = 129.075]; nonusers: M = 495.669 [SD = 142.241]; p = .414), nor movement duration (users: M = 759.602 [SD = 135.615]; nonusers: M = 737.759 [SD = 242.288]; p = .734). These data are shown in Figure 1b.
3.2. Sensor‐level analysis
Sensor‐level time‐frequency analyses across all participants revealed a significant peri‐movement β‐ERD response in sensors near the left primary motor cortex (Figure 2a). This peri‐movement β‐ERD had a bandwidth of 16–24 Hz, began around 500 ms prior to movement onset, and lasted for approximately 1000 ms (i.e., −500 to 500 ms; p < .0001, corrected). It is important to note that a robust synchronization was evident after the ERD (i.e., the post‐movement beta rebound; PMBR). This PMBR was not subjected to source reconstruction because it was tightly yoked to the termination of movement, and our study focused on motor planning and execution processes.
FIGURE 2.
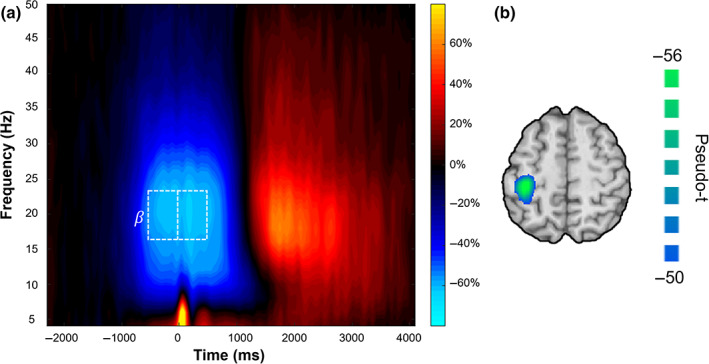
Time‐frequency spectrogram and source reconstruction. (a) Grand‐averaged time‐frequency spectrogram across all participants from a sensor near the left sensorimotor cortex (i.e., MEG0432). Time (ms) is displayed on the x‐axis with frequency (Hz) on the y‐axis. Signal power is expressed as a percent change from baseline. Before and during movement, there was a strong beta event‐related desynchronization (ERD; 16–24 Hz), followed by a post‐movement beta rebound (PMBR). The white box displays the two time‐frequency bins selected for beamforming analyses (planning: −500 to 0 ms; execution: 0–500 ms; both at 16–24 Hz) to isolate the motor planning and execution phases. Note that we did not image the PMBR because it is tightly linked to motor termination, and our study was focused on motor planning and execution processes. (b) Grand‐averaged beamformer image across both time windows and groups showed that the beta ERD was strongest in the contralateral primary motor cortices. The peak voxel was located at the same coordinates in the grand‐averaged image computed using the planning and execution images separately, as well as combined. Voxel time series data were extracted using the peak voxel in this grand‐averaged map.
3.3. Beamforming and virtual sensor analysis
To identify the anatomical regions generating the β‐ERD response, we imaged the significant time‐frequency window from the sensor‐level analysis. Since our baseline was only 500 ms long and we were interested in distinguishing motor planning from execution related processing, we imaged the β‐ERD in two separate windows (i.e., motor planning = −500 to 0 ms; execution = 0 to 500 ms). This revealed a robust response in the contralateral primary motor cortex across both groups and time windows, with the peak voxel of activation occurring at the same voxel coordinates in both the planning and execution windows (Figure 2b). To examine the temporal dynamics, virtual sensor time series were extracted from the peak voxel in the grand‐averaged image per participant. These time series were probed for group differences per time window using a mixed‐model ANOVA (Figure 3). The results revealed a group‐by‐window interaction, such that users had stronger beta oscillations in the execution phase relative to nonusers, while the two groups did not differ during motor planning (F 1,39 = 6.612, p = .014). There was also a main effect of window such that independent of group, the participants had stronger beta oscillations in the execution phase (F 1,39 = 11.041, p = .002). Of note, there was no main effect of group on the overall strength of beta oscillations (F 1,39 = 1.6255, p = .210). Further, there were no group differences in the latency of the primary motor responses (β‐ERD latency: F 1,39 = 1.588, p = .215; PMBR latency: F 1,39 = 1.012, p = .321) (Figure 2a).
FIGURE 3.
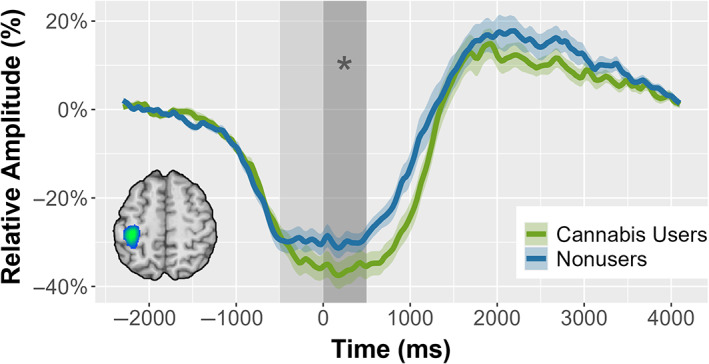
Time series of the β‐ERD response in the primary motor cortex. Time series of the peak β‐ERD voxel, separated by group. The shaded area indicates the two β‐ERD time windows, with the motor planning window (−500 to 0 ms) in light gray and the motor execution window (0–500 ms) shown in darker gray. The asterisk (*) denotes the significant main effect of window (p = .002) and group‐by‐window interaction (p = .014). The inset in the bottom left shows the grand‐averaged β‐ERD beamformer image; the time series was extracted from the peak voxel, which was within the primary motor cortex contralateral to movement.
Next, we conducted whole‐brain analyses to determine whether brain regions beyond the primary motor cortex differed between groups. Specifically, we conducted a whole‐brain mixed‐model ANOVA, with group and time window as factors. This revealed a group‐by‐window interaction effect across multiple brain regions, with significant peaks in the left superior parietal cortices, right inferior frontal gyrus, and the right posterior insula (p < .001; Figure 4), in addition to the primary motor cortices. In each of these regions, users exhibited stronger beta oscillations (i.e., more robust decreases in beta power) during the movement execution phase relative to nonusers, with the beta oscillatory amplitude not differing between groups during the planning phase. Additionally, there were significant main effects of window in all three peaks (parietal: F 1,38 = 4.144, p = .049; frontal: F 1,38 = 52.010, p < .001; insula: F 1,38 = 74.068 p < .001) such that independent of group, participants exhibited stronger beta oscillations in the execution phase relative to the planning phase.
FIGURE 4.
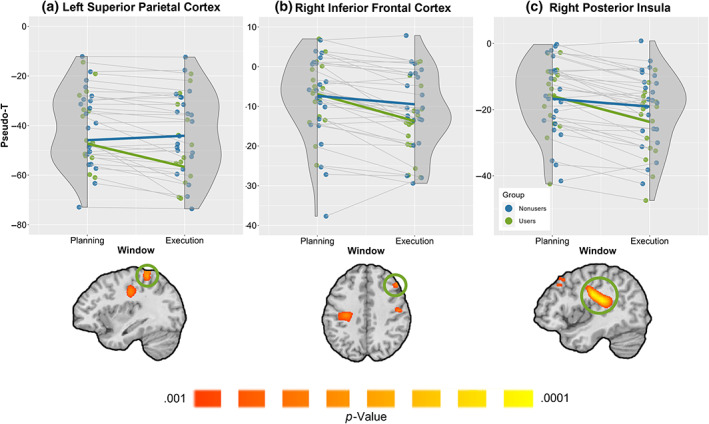
Group‐by‐window interaction effect. Cannabis users exhibited stronger β‐ERD responses during the execution phase compared to nonusers in the (a) left superior parietal cortices, (b) right inferior frontal cortices, (c) right posterior insula, and the left primary motor cortices (p < .001, corrected). The groups did not differ during the motor planning phase. (Top) Rain cloud plots showing peak voxel pseudo‐t values per participant from the region with the green circle in the images below each plot. Users are shown in green and nonusers in blue. The x‐axis is separated into the planning and execution windows (i.e., −500 to 0 and 0 to 500, respectively), and the y‐axis displays pseudo‐t values. (Bottom) Statistical maps showing the group‐by‐window interaction effect. In addition to the three regions noted above, significant clusters were found in the right (ipsilateral) precentral gyrus and the left (contralateral) precentral gyrus.
4. DISCUSSION
In this study, we used a motor sequence paradigm and MEG imaging to study the effect of regular cannabis use on complex motor control. The strength of the motor β‐ERD has been shown to be modulated by development (Fung et al., 2022; Gaetz et al., 2010; Heinrichs‐Graham et al., 2018) and to increase with age during adulthood (Heinrichs‐Graham & Wilson, 2016; Rossiter et al., 2014; Trevarrow et al., 2019; Wilson et al., 2010). The motor‐related β‐ERD is also known to be altered by healthy aging, as well as several pathologies including cerebral palsy, stroke, Parkinson's disease, neuroHIV, psychosis, and amyotrophic lateral sclerosis (Bizovičar et al., 2014; Heinrichs‐Graham, Santamaria, et al., 2017; Heinrichs‐Graham, Wilson, et al., 2014; McCusker et al., 2021; Peter et al., 2022; Shiner et al., 2015; Spooner et al., 2023; Wilson et al., 2013, 2019; Wilson, Slason, et al., 2011). Our key findings expand upon this literature by demonstrating that regular cannabis users exhibit stronger motor‐related β‐ERD responses in the primary motor cortices and multiple higher‐order motor regions compared to nonusers. Further, these differences were observed only during the motor execution phase and not motor planning. These findings and their implications are discussed below.
Our most interesting findings were likely the altered dynamics in the primary motor cortex of the cannabis users. Specifically, cannabis users exhibited a significant increase in beta oscillatory strength during the motor execution window, which appeared to emerge shortly before movement onset and then was sustained through the entire execution period. Such data indicates heightened activity within the primary motor cortex, which is known to be the main output center for the cortical‐spinal tract. While we did not observe any motor performance differences between groups, numerous past studies have shown that the amplitude of the β‐ERD response is closely related to motor performance (Kurz et al., 2014, 2020; Spooner et al., 2021; Wiesman et al., 2020; Wilson, Fleischer, et al., 2011). Additionally, studies have shown that patients with Parkinson's disease, as well as cerebral palsy, exhibit weakened β‐ERD responses (Heinrichs‐Graham, Wilson, et al., 2014; Trevarrow et al., 2022). The strength of such beta oscillations is at least partially driven by activity in γ‐aminobutyric acid (GABA) interneurons, with MEG studies linking increased sensorimotor cortex GABA concentration with stronger β‐ERDs (Hall et al., 2011; Muthukumaraswamy et al., 2013). Further, reduced GABA concentrations in the motor cortex of Parkinson's patients has been shown to predict increased symptom severity (van Nuland et al., 2020); thus, it follows that as symptoms become more severe, the corresponding decrease in GABA concentration may play a role in the reduction of β‐ERD strength. Further, using the same sequencing task as in our study, Heinrichs‐Graham and Wilson (2016) demonstrated stronger β‐ERD responses in healthy aging. These increases in β‐ERD strength were thought to be compensatory in nature as the baseline beta amplitude was also significantly elevated in older adults, thereby requiring a stronger β‐ERD to reach a similar level of beta prior to initiating movement (Heinrichs‐Graham & Wilson, 2016; Wilson et al., 2014). Thus, in our study, such increased beta oscillatory activity during execution may have enabled the users to maintain performance levels similar to controls. Future studies using more complex motor tasks may be better able to discern such effects and identify declines in performance related to cannabis use, although caution is warranted as differences in performance alone are frequently associated with oscillatory differences in the absence of neuropathology.
Motor‐related neural activity involves the coordinated efforts of the primary motor cortices and several higher‐order motor regions (Ball et al., 1999; Georgopoulos, 1991; Heinrichs‐Graham et al., 2016; Sakata et al., 2017). Some key higher‐order motor regions include those in the frontal cortex, supplementary motor area (SMA), premotor, and parietal regions (Ricci et al., 2019; Wendiggensen et al., 2022). Though literature regarding the contributions of the insular cortex during motor actions is scant, this region has been implicated in various aspects of motor planning and execution (Beurze et al., 2007; Caliandro et al., 2021; Olausson et al., 2002; Sakata et al., 2017). Results from our whole‐brain analyses showed that cannabis users exhibited stronger beta oscillations relative to nonusers during the execution phase in the frontal, parietal, motor, and insular cortices. In contrast, the two groups did not differ during the motor planning phase. These results suggest that users must recruit more higher‐order neural resources during the execution phase to accurately perform the motor sequencing task. In regard to the mechanisms, local GABA concentrations have been shown to play a role in generating beta oscillations in the motor cortex (Cheyne, 2013; Gaetz et al., 2011). Studies investigating healthy aging have linked increases in GABAergic inhibition with stronger β‐ERD responses (Rossiter et al., 2014), and others have linked cannabidiol, the primary non‐psychoactive component of the Cannabis sativa plant (Bielawiec et al., 2020), with increases in GABAergic transmission (Kaplan et al., 2017; Musella et al., 2009; Pretzsch et al., 2019). Thus, it follows that regular consumption of cannabis may lead to increased GABAergic transmission, which may be responsible for these stronger beta oscillations across multiple cortical regions serving motor control. These types of region‐specific oscillatory changes in chronic cannabis users have been demonstrated previously by our laboratory and others (Arif et al., 2021; Schantell et al., 2022; Springer et al., 2021; Weyrich et al., 2023), but our current findings are the first to extend this literature into motor‐related beta oscillations.
Taken together, our results demonstrate that though regular cannabis users are able to perform the current motor sequencing task at the same level as nonuser controls, the two groups are quite different neurologically. These differences may reflect compensatory processing or be precursors of behavioral deficits that may emerge in the future (Rangel‐Pacheco et al., 2021). These compensatory mechanisms, though adequate for this relatively simple sequencing task, may break down in real‐world situations where more complex motor control is needed. For example, presumably, nonusers would exhibit stronger beta oscillations during more complex motor tasks (Heinrichs‐Graham & Wilson, 2015) and such increases may not be possible in users due to limitations in the dynamic range of cortical beta activity (Heinrichs‐Graham & Wilson, 2016; Wilson et al., 2014). More broadly, compensatory activity through increased neural oscillations would likely be governed by task difficulty and may break down in more real‐life motor sequence scenarios.
Before closing, it is important to acknowledge the limitations of this study, as well as directions for future work. Even in states where recreational cannabis is legal, government regulation has been largely unable to control the potency or percentage of tetrahydrocannabinol (THC) in cannabis (Pacula et al., 2022), and thus we were unable to control for the amount, isoform, and method of consumption of THC in our user group. To mitigate this limitation, we had all participants complete a detailed questionnaire and undergo structural clinical interviews about their use patterns. Future studies could consider a subgroup approach that would allow group comparisons among those using different types and cannabis doses, but this would necessitate much larger enrollment numbers, which could create feasibility concerns. Future studies may also benefit from using tasks with higher cognitive demands in an attempt to create a more “naturalistic” design to elucidate the dynamics of the compensatory mechanisms described in this work, and to identify if and at what point these compensatory processes become exhausted. The motor complexity task used in the current study was only moderately demanding, possibly explaining the largely equivalent behavioral performance across the two groups. Future studies should also use other motor tasks that are known to elicit motor‐related gamma responses (e.g., Spooner & Wilson, 2022), as such high‐frequency responses may be associated with unique deficits in cannabis users (e.g., Arif et al., 2021). Finally, caution is warranted in considering the underlying mechanisms. While our interpretation of the results focuses on the potential impact of cannabis use on the neural oscillations serving motor control, it is also possible that these differences are more indicative of risk factors for developing cannabis use disorders. Determining the precise directionality will require longitudinal designs, with cannabis‐naïve individuals being scanned prior to initiating cannabis use and after having regularly used for multiple years, and should be a goal for future work. Despite these limitations, the current study clearly indicates that regular cannabis use is associated with alterations across multiple brain regions involved in motor control and that these alterations emerge most clearly during the execution of motor sequences and not their planning.
FUNDING INFORMATION
This work was supported by the National Institutes of Health (grant numbers R01‐DA047828, R03‐DA041917, R01‐DA056223, R01‐MH103220, R01‐MH116782, R01‐MH118013, and P20‐GM144641 to Wilson, F31‐DA056296 and R36‐DA059323 to Schantell, F30‐AG076259 to Springer, and F30‐MH130150 to Killanin).
CONFLICT OF INTEREST STATEMENT
The authors of this manuscript acknowledge no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We want to thank the participants for volunteering to participate in the study and our staff and local collaborators for contributing to the work.
Ward, T. W. , Springer, S. D. , Schantell, M. , John, J. A. , Horne, L. K. , Coutant, A. T. , Okelberry, H. J. , Willett, M. P. , Johnson, H. J. , Killanin, A. D. , Heinrichs‐Graham, E. , & Wilson, T. W. (2023). Regular cannabis use alters the neural dynamics serving complex motor control. Human Brain Mapping, 44(18), 6511–6522. 10.1002/hbm.26527
DATA AVAILABILITY STATEMENT
The data used in this article will be made publicly available through the COINS framework at the completion of the study (https://coins.trendscenter.org/).
REFERENCES
- Anderson, B. M. , Rizzo, M. , Block, R. I. , Pearlson, G. D. , & O'Leary, D. S. (2010). Sex, drugs, and cognition: Effects of marijuana. Journal of Psychoactive Drugs, 42(4), 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif, Y. , Wiesman, A. I. , Christopher‐Hayes, N. J. , & Wilson, T. W. (2021). Aberrant inhibitory processing in the somatosensory cortices of cannabis‐users. Journal of Psychopharmacology (Oxford, England), 35(11), 1356–1364. 10.1177/02698811211050557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin, D. J. , Heinrichs‐Graham, E. , Gehringer, J. E. , Zabad, R. , Wilson, T. W. , & Kurz, M. J. (2017). Altered sensorimotor cortical oscillations in individuals with multiple sclerosis suggests a faulty internal model. Human Brain Mapping, 38(8), 4009–4018. 10.1002/hbm.23644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, T. , Schreiber, A. , Feige, B. , Wagner, M. , Lücking, C. H. , & Kristeva‐Feige, R. (1999). The role of higher‐order motor areas in voluntary movement as revealed by high‐resolution EEG and fMRI. NeuroImage, 10(6), 682–694. 10.1006/nimg.1999.0507 [DOI] [PubMed] [Google Scholar]
- Beurze, S. M. , de Lange, F. P. , Toni, I. , & Medendorp, W. P. (2007). Integration of target and effector information in the human brain during reach planning. Journal of Neurophysiology, 97(1), 188–199. 10.1152/jn.00456.2006 [DOI] [PubMed] [Google Scholar]
- Bielawiec, P. , Harasim‐Symbor, E. , & Chabowski, A. (2020). Phytocannabinoids: Useful drugs for the treatment of obesity? Special focus on cannabidiol. Frontiers in Endocrinology, 11, 114. 10.3389/fendo.2020.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizovičar, N. , Dreo, J. , Koritnik, B. , & Zidar, J. (2014). Decreased movement‐related beta desynchronization and impaired post‐movement beta rebound in amyotrophic lateral sclerosis. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 125(8), 1689–1699. 10.1016/j.clinph.2013.12.108 [DOI] [PubMed] [Google Scholar]
- Bosker, W. M. , Karschner, E. L. , Lee, D. , Goodwin, R. S. , Hirvonen, J. , Innis, R. B. , Theunissen, E. L. , Kuypers, K. P. C. , Huestis, M. A. , & Ramaekers, J. G. (2013). Psychomotor function in chronic daily cannabis smokers during sustained abstinence. PLoS One, 8(1), e53127. 10.1371/journal.pone.0053127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliandro, P. , Menegaz, G. , Iacovelli, C. , Conte, C. , Reale, G. , Calabresi, P. , & Storti, S. F. (2021). Connectivity modulations induced by reach&grasp movements: A multidimensional approach. Scientific Reports, 11, 23097. 10.1038/s41598-021-02458-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne, D. O. (2013). MEG studies of sensorimotor rhythms: A review. Experimental Neurology, 245, 27–39. 10.1016/j.expneurol.2012.08.030 [DOI] [PubMed] [Google Scholar]
- Crane, N. A. , Schuster, R. M. , Fusar‐Poli, P. , & Gonzalez, R. (2013). Effects of cannabis on neurocognitive functioning: Recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review, 23(2), 117–137. 10.1007/s11065-012-9222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders, F. C. (1969). On the speed of mental processes. Acta Psychologica, 30, 412–431. 10.1016/0001-6918(69)90065-1 [DOI] [PubMed] [Google Scholar]
- D'Souza, D. C. , Braley, G. , Blaise, R. , Vendetti, M. , Oliver, S. , Pittman, B. , Ranganathan, M. , Bhakta, S. , Zimolo, Z. , Cooper, T. , & Perry, E. (2008). Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Δ‐9‐tetrahydrocannabinol in humans. Psychopharmacology, 198(4), 587–603. 10.1007/s00213-007-1042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, D. C. , Ranganathan, M. , Braley, G. , Gueorguieva, R. , Zimolo, Z. , Cooper, T. , Perry, E. , & Krystal, J. (2008). Blunted psychotomimetic and amnestic effects of Δ‐9‐tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 33(10), 2505–2516. 10.1038/sj.npp.1301643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duperrouzel, J. C. , Granja, K. , Pacheco‐Colón, I. , & Gonzalez, R. (2020). Adverse effects of cannabis use on neurocognitive functioning: A systematic review of meta‐ analytic studies. Journal of Dual Diagnosis, 16(1), 43–57. 10.1080/15504263.2019.1626030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury, C. M. , Heinrichs‐Graham, E. , Lord, G. H. , Drincic, A. T. , Desouza, C. V. , & Wilson, T. W. (2019). Altered motor dynamics in type 1 diabetes modulate behavioral performance. NeuroImage: Clinical, 24, 101977. 10.1016/j.nicl.2019.101977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, M. D. (2004). Permutation methods: A basis for exact inference. Statistical Science, 19, 676–685. [Google Scholar]
- Everitt, B. J. , & Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience, 8(11), 1481–1489. 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Ferraro, D. P. (1980). Acute effects of marijuana on human memory and cognition. NIDA Research Monograph, 31, 98–119. [PubMed] [Google Scholar]
- Fung, M. H. , Heinrichs‐Graham, E. , Taylor, B. K. , Frenzel, M. R. , Eastman, J. A. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , & Wilson, T. W. (2022). The development of sensorimotor cortical oscillations is mediated by pubertal testosterone. NeuroImage, 264, 119745. 10.1016/j.neuroimage.2022.119745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz, W. , Edgar, J. C. , Wang, D. J. , & Roberts, T. P. L. (2011). Relating MEG measured motor cortical oscillations to resting γ‐aminobutyric acid (GABA) concentration. NeuroImage, 55(2), 616–621. 10.1016/j.neuroimage.2010.12.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz, W. , Macdonald, M. , Cheyne, D. , & Snead, O. C. (2010). Neuromagnetic imaging of movement‐related cortical oscillations in children and adults: Age predicts post‐movement beta rebound. NeuroImage, 51(2), 792–807. 10.1016/j.neuroimage.2010.01.077 [DOI] [PubMed] [Google Scholar]
- Georgopoulos, A. P. (1991). Higher order motor control. Annual Review of Neuroscience, 14(1), 361–377. 10.1146/annurev.ne.14.030191.002045 [DOI] [PubMed] [Google Scholar]
- Goldstein, R. Z. , & Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry, 159(10), 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. , Kujala, J. , Hamalainen, M. , Timmermann, L. , Schnitzler, A. , & Salmelin, R. (2001). Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A., 98(2), 694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, S. D. , Stanford, I. M. , Yamawaki, N. , McAllister, C. J. , Rönnqvist, K. C. , Woodhall, G. L. , & Furlong, P. L. (2011). The role of GABAergic modulation in motor function related neuronal network activity. NeuroImage, 56(3), 1506–1510. 10.1016/j.neuroimage.2011.02.025 [DOI] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Arpin, D. J. , & Wilson, T. W. (2016). Cue‐related temporal factors modulate movement‐related beta oscillatory activity in the human motor circuit. Journal of Cognitive Neuroscience, 28(7), 1039–1051. 10.1162/jocn_a_00948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Kurz, M. J. , Becker, K. M. , Santamaria, P. M. , Gendelman, H. E. , & Wilson, T. W. (2014). Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson's disease: A pharmaco‐magnetoencephalography study. Journal of Neurophysiology, 112(7), 1739–1747. 10.1152/jn.00383.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Kurz, M. J. , Gehringer, J. E. , & Wilson, T. W. (2017). The functional role of post‐movement beta oscillations in motor termination. Brain Structure and Function, 222(7), 3075–3086. 10.1007/s00429-017-1387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , McDermott, T. J. , Mills, M. S. , Wiesman, A. I. , Wang, Y.‐P. , Stephen, J. M. , Calhoun, V. D. , & Wilson, T. W. (2018). The lifespan trajectory of neural oscillatory activity in the motor system. Developmental Cognitive Neuroscience, 30, 159–168. 10.1016/j.dcn.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Santamaria, P. M. , Gendelman, H. E. , & Wilson, T. W. (2017). The cortical signature of symptom laterality in Parkinson's disease. NeuroImage: Clinical, 14, 433–440. 10.1016/j.nicl.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Taylor, B. K. , Wang, Y.‐P. , Stephen, J. M. , Calhoun, V. D. , & Wilson, T. W. (2020). Parietal oscillatory dynamics mediate developmental improvement in motor performance. Cerebral Cortex (New York, NY: 1991), 30(12), 6405–6414. 10.1093/cercor/bhaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , & Wilson, T. W. (2015). Coding complexity in the human motor circuit. Human Brain Mapping, 36(12), 5155–5167. 10.1002/hbm.23000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , & Wilson, T. W. (2016). Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. NeuroImage, 134, 514–521. 10.1016/j.neuroimage.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Wilson, T. W. , Santamaria, P. M. , Heithoff, S. K. , Torres‐Russotto, D. , Hutter‐Saunders, J. A. L. , Estes, K. A. , Meza, J. L. , Mosley, R. L. , & Gendelman, H. E. (2014). Neuromagnetic evidence of abnormal movement‐related Beta desynchronization in Parkinson's disease. Cerebral Cortex (New York, NY), 24(10), 2669–2678. 10.1093/cercor/bht121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros, I. , Miquel, L. , Blithikioti, C. , Nuño, L. , Rubio Ballester, B. , Grechuta, K. , Gual, A. , Balcells‐Oliveró, M. , & Verschure, P. (2019). Motor adaptation impairment in chronic cannabis users assessed by a visuomotor rotation task. Journal of Clinical Medicine, 8(7), 1049. 10.3390/jcm8071049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand, A. , Singh, K. D. , Holliday, I. E. , Furlong, P. L. , & Barnes, G. R. (2005). A new approach to neuroimaging with magnetoencephalography. Human Brain Mapping, 25(2), 199–211. 10.1002/hbm.20102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, R. M. , Wilson, T. W. , & Kurz, M. J. (2019). Hand motor actions of children with cerebral palsy are associated with abnormal sensorimotor cortical oscillations. Neurorehabilitation and Neural Repair, 33(12), 1018–1028. 10.1177/1545968319883880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz, M. T. , Gaetz, W. C. , Bostan, A. C. , & Cheyne, D. (2006). Post‐movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. NeuroImage, 32(3), 1281–1289. 10.1016/j.neuroimage.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Kaplan, J. S. , Stella, N. , Catterall, W. A. , & Westenbroek, R. E. (2017). Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proceedings of the National Academy of Sciences, 114(42), 11229–11234. 10.1073/pnas.1711351114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach, C. K. , & Gander, P. E. (2016). The demodulated band transform. Journal of Neuroscience Methods, 261, 135–154. 10.1016/j.jneumeth.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon, E. , Kuhns, L. , & Cousijn, J. (2021). The short‐term and long‐term effects of cannabis on cognition: Recent advances in the field. Current Opinion in Psychology, 38, 49–55. 10.1016/j.copsyc.2020.07.005 [DOI] [PubMed] [Google Scholar]
- Kurz, M. J. , Becker, K. M. , Heinrichs‐Graham, E. , & Wilson, T. W. (2014). Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Developmental Medicine and Child Neurology, 56(11), 1072–1077. 10.1111/dmcn.12513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz, M. J. , Bergwell, H. , Spooner, R. , Baker, S. , Heinrichs‐Graham, E. , & Wilson, T. W. (2020). Motor beta cortical oscillations are related with the gait kinematics of youth with cerebral palsy. Annals of Clinical and Translational Neurology, 7(12), 2421–2432. 10.1002/acn3.51246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, G. D. , Cowan, W. B. , & Davis, K. A. (1984). On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology. Human Perception and Performance, 10(2), 276–291. 10.1037//0096-1523.10.2.276 [DOI] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. [DOI] [PubMed] [Google Scholar]
- McCusker, M. C. , Wiesman, A. I. , Spooner, R. K. , Santamaria, P. M. , McKune, J. , Heinrichs‐Graham, E. , & Wilson, T. W. (2021). Altered neural oscillations during complex sequential movements in patients with Parkinson's disease. NeuroImage: Clinical, 32, 102892. 10.1016/j.nicl.2021.102892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. , Schleifer, L. , Richards, J. B. , & de Wit, H. (2003). Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology, 28(7), 7–1365. 10.1038/sj.npp.1300176 [DOI] [PubMed] [Google Scholar]
- McFarquhar, M. , McKie, S. , Emsley, R. , Suckling, J. , Elliott, R. , & Williams, S. (2016). Multivariate and repeated measures (MRM): A new toolbox for dependent and multimodal group‐level neuroimaging data. NeuroImage, 132, 373–389. 10.1016/j.neuroimage.2016.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik, J. , Kahler, C. W. , Reynolds, B. , McGeary, J. E. , Monti, P. M. , Haney, M. , de Wit, H. , & Rohsenow, D. J. (2012). Balanced placebo design with marijuana: Pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology, 223(4), 489–499. 10.1007/s00213-012-2740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musella, A. , de Chiara, V. , Rossi, S. , Prosperetti, C. , Bernardi, G. , Maccarrone, M. , & Centonze, D. (2009). TRPV1 channels facilitate glutamate transmission in the striatum. Molecular and Cellular Neuroscience, 40(1), 89–97. 10.1016/j.mcn.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy, S. D. , Myers, J. F. M. , Wilson, S. J. , Nutt, D. J. , Lingford‐Hughes, A. , Singh, K. D. , & Hamandi, K. (2013). The effects of elevated endogenous GABA levels on movement‐related network oscillations. NeuroImage, 66, 36–41. 10.1016/j.neuroimage.2012.10.054 [DOI] [PubMed] [Google Scholar]
- Ng, T. H. B. , Sowman, P. F. , Brock, J. , & Johnson, B. W. (2011). Premovement brain activity in a bimanual load‐lifting task. Experimental Brain Research, 208(2), 189–201. 10.1007/s00221-010-2470-5 [DOI] [PubMed] [Google Scholar]
- Olausson, H. , Lamarre, Y. , Backlund, H. , Morin, C. , Wallin, B. G. , Starck, G. , Ekholm, S. , Strigo, I. , Worsley, K. , Vallbo, A. B. , & Bushnell, M. C. (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience, 5(9), 900–904. 10.1038/nn896 [DOI] [PubMed] [Google Scholar]
- Pacula, R. , Pessar, S. , Zhu, J. , Kritikos, A. , & Smart, R. (2022). Federal regulation of cannabis for public health in the United States. Schaeffer Center White Paper Series, 213, 810–8554. 10.25549/FBEW-6Z03 [DOI] [Google Scholar]
- Papp, N. , & Ktonas, P. (1977). Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomedical Sciences Instrumentation, 13, 135–145. [PubMed] [Google Scholar]
- Patrick, M. E. , Schulenberg, J. E. , Miech, R. A. , Johnston, L. D. , O'Malley, P. M. , & Bachman, J. G. (2022). Monitoring the future panel study annual report: National data on substance use among adults ages 19 to 60, 1976–2021 (Monitoring the Future Monograph Series, p. 193). University of Michigan Institute for Social Research; https://monitoringthefuture.org/wp‐content/uploads/2022/09/mtfpanelreport2022.pdf [Google Scholar]
- Peter, J. , Ferraioli, F. , Mathew, D. , George, S. , Chan, C. , Alalade, T. , Salcedo, S. A. , Saed, S. , Tatti, E. , Quartarone, A. , & Ghilardi, M. F. (2022). Movement‐related beta ERD and ERS abnormalities in neuropsychiatric disorders. Frontiers in Neuroscience, 16, 1045715. 10.3389/fnins.2022.1045715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller, G. , & Lopes da Silva, F. H. (1999). Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology, 110(11), 1842–1857. 10.1016/S1388-2457(99)00141-8 [DOI] [PubMed] [Google Scholar]
- Pillay, S. S. , Rogowska, J. , Kanayama, G. , Gruber, S. , Simpson, N. , Pope, H. G. , & Yurgelun‐Todd, D. A. (2008). Cannabis and motor function: FMRI changes following 28 days of discontinuation. Experimental and Clinical Psychopharmacology, 16(1), 22–32. 10.1037/1064-1297.16.1.22 [DOI] [PubMed] [Google Scholar]
- Poline, J. B. , Worsley, K. J. , Holmes, A. P. , Frackowiak, R. S. , & Friston, K. J. (1995). Estimating smoothness in statistical parametric maps: Variability of p values. Journal of Computer Assisted Tomography, 19(5), 788–796. 10.1097/00004728-199509000-00017 [DOI] [PubMed] [Google Scholar]
- Pretzsch, C. M. , Freyberg, J. , Voinescu, B. , Lythgoe, D. , Horder, J. , Mendez, M. A. , Wichers, R. , Ajram, L. , Ivin, G. , Heasman, M. , Edden, R. A. E. , Williams, S. , Murphy, D. G. M. , Daly, E. , & McAlonan, G. M. (2019). Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo‐controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology, 44(8), 8–1405. 10.1038/s41386-019-0333-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel‐Pacheco, A. , Lew, B. J. , Schantell, M. D. , Frenzel, M. R. , Eastman, J. A. , Wiesman, A. I. , & Wilson, T. W. (2021). Altered fronto‐occipital connectivity during visual selective attention in regular cannabis users. Psychopharmacology, 238(5), 1351–1361. 10.1007/s00213-020-05717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci, S. , Tatti, E. , Mehraram, R. , Panday, P. , & Ghilardi, M. F. (2019). Beta band frequency differences between motor and frontal cortices in reaching movements. IEEE International Conference on Rehabilitation Robotics: [Proceedings], 2019, 1254–1259. 10.1109/ICORR.2019.8779373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter, H. E. , Davis, E. M. , Clark, E. V. , Boudrias, M.‐H. , & Ward, N. S. (2014). Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. NeuroImage, 91(100), 360–365. 10.1016/j.neuroimage.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata, H. , Itoh, K. , Suzuki, Y. , Nakamura, K. , Watanabe, M. , Igarashi, H. , & Nakada, T. (2017). Slow accumulations of neural activities in multiple cortical regions precede self‐initiation of movement: An event‐related fMRI study. ENeuro, 4(5), ENEURO.0183‐17.2017. 10.1523/ENEURO.0183-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell, M. , Springer, S. D. , Arif, Y. , Sandal, M. E. , Willett, M. P. , Johnson, H. J. , Okelberry, H. J. , O'Neill, J. L. , May, P. E. , Bares, S. H. , & Wilson, T. W. (2022). Regular cannabis use modulates the impact of HIV on the neural dynamics serving cognitive control. Journal of Psychopharmacology (Oxford, England), 36(12), 1324–1337. 10.1177/02698811221138934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner, A. M. , & Dunn, M. E. (2012). Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta‐analysis. Experimental and Clinical Psychopharmacology, 20(5), 420–429. 10.1037/a0029117 [DOI] [PubMed] [Google Scholar]
- Shiner, C. T. , Tang, H. , Johnson, B. W. , & McNulty, P. A. (2015). Cortical beta oscillations and motor thresholds differ across the spectrum of post‐stroke motor impairment, a preliminary MEG and TMS study. Brain Research, 1629, 26–37. 10.1016/j.brainres.2015.09.037 [DOI] [PubMed] [Google Scholar]
- Spooner, R. K. , Arif, Y. , Taylor, B. K. , & Wilson, T. W. (2021). Movement‐related gamma synchrony differentially predicts behavior in the presence of visual interference across the lifespan. Cerebral Cortex (New York, NY: 1991), 31(11), 5056–5066. 10.1093/cercor/bhab141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner, R. K. , Taylor, B. K. , Ahmad, I. M. , Dyball, K. , Emanuel, K. , O'Neill, J. , Kubat, M. , Swindells, S. , Fox, H. S. , Bares, S. H. , Stauch, K. L. , Zimmerman, M. C. , & Wilson, T. W. (2023). Mitochondrial redox environments predict sensorimotor brain‐behavior dynamics in adults with HIV. Brain, Behavior, and Immunity, 107, 265–275. 10.1016/j.bbi.2022.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner, R. K. , Taylor, B. K. , Ahmad, I. M. , Dyball, K. N. , Emanuel, K. , Fox, H. S. , Stauch, K. L. , Zimmerman, M. C. , & Wilson, T. W. (2021). Neural oscillatory activity serving sensorimotor control is predicted by superoxide‐sensitive mitochondrial redox environments. Proceedings of the National Academy of Sciences of the United States of America, 118(43), e2104569118. 10.1073/pnas.2104569118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner, R. K. , & Wilson, T. W. (2022). Cortical theta–gamma coupling governs the adaptive control of motor commands. Brain Communications, 4(6), fcac249. 10.1093/braincomms/fcac249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner, R. K. , & Wilson, T. W. (2023). Spectral specificity of gamma‐frequency transcranial alternating current stimulation over motor cortex during sequential movements. Cerebral Cortex (New York, NY: 1991), 33(9), 5347–5360. 10.1093/cercor/bhac423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, S. D. , Spooner, R. K. , Schantell, M. , Arif, Y. , Frenzel, M. R. , Eastman, J. A. , & Wilson, T. W. (2021). Regular recreational Cannabis users exhibit altered neural oscillatory dynamics during attention reorientation. Psychological Medicine, 1–10, 1205–1214. 10.1017/S0033291721002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . (2020). Key substance use and mental health indicators in the United States: Results from the 2020. National Survey on Drug Use and Health, 156, 2. [Google Scholar]
- Taulu, S. , & Simola, J. (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology, 51(7), 1759–1768. 10.1088/0031-9155/51/7/008 [DOI] [PubMed] [Google Scholar]
- Trevarrow, M. P. , Kurz, M. J. , McDermott, T. J. , Wiesman, A. I. , Mills, M. S. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , & Wilson, T. W. (2019). The developmental trajectory of sensorimotor cortical oscillations. NeuroImage, 184, 455–461. 10.1016/j.neuroimage.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarrow, M. P. , Reelfs, A. , Baker, S. E. , Hoffman, R. M. , Wilson, T. W. , & Kurz, M. J. (2022). Spinal cord microstructural changes are connected with the aberrant sensorimotor cortical oscillatory activity in adults with cerebral palsy. Scientific Reports, 12, 4807. 10.1038/s41598-022-08741-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis, C. , Ince, N. F. , Leuthold, A. C. , & Pellizzer, G. (2010). Beta‐band activity during motor planning reflects response uncertainty. The Journal of Neuroscience, 30(34), 11270–11277. 10.1523/JNEUROSCI.6026-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo, M. A. , & Ilmoniemi, R. J. (1997). Signal‐space projection method for separating MEG or EEG into components. Medical and Biological Engineering and Computing, 35, 135–140. [DOI] [PubMed] [Google Scholar]
- van Nuland, A. J. M. , den Ouden, H. E. M. , Zach, H. , Dirkx, M. F. M. , van Asten, J. J. A. , Scheenen, T. W. J. , Toni, I. , Cools, R. , & Helmich, R. C. (2020). GABAergic changes in the thalamocortical circuit in Parkinson's disease. Human Brain Mapping, 41(4), 1017–1029. 10.1002/hbm.24857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen, B. D. , van Drongelen, W. , Yuchtman, M. , & Suzuki, A. (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng, 44(9), 867–880. 10.1109/10.623056 [DOI] [PubMed] [Google Scholar]
- Wendiggensen, P. , Adelhöfer, N. , Jamous, R. , Mückschel, M. , Takacs, A. , Frings, C. , Münchau, A. , & Beste, C. (2022). Processing of embedded response plans is modulated by an interplay of frontoparietal theta and beta activity. Journal of Neurophysiology, 128(3), 543–555. 10.1152/jn.00537.2021 [DOI] [PubMed] [Google Scholar]
- Weyrich, L. , Arif, Y. , Schantell, M. , Johnson, H. J. , Willett, M. P. , Okelberry, H. J. , & Wilson, T. W. (2023). Altered functional connectivity and oscillatory dynamics in polysubstance and cannabis only users during visuospatial processing. Psychopharmacology, 240, 769–783. 10.1007/s00213-023-06318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant graphics for data analysis. Springer‐Verlag; https://ggplot2.tidyverse.org [Google Scholar]
- Wiesman, A. I. , Christopher‐Hayes, N. J. , Eastman, J. A. , Heinrichs‐Graham, E. , & Wilson, T. W. (2021). Response certainty during bimanual movements reduces gamma oscillations in primary motor cortex. NeuroImage, 224, 117448. 10.1016/j.neuroimage.2020.117448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman, A. I. , Koshy, S. M. , Heinrichs‐Graham, E. , & Wilson, T. W. (2020). Beta and gamma oscillations index cognitive interference effects across a distributed motor network. NeuroImage, 213, 116747. 10.1016/j.neuroimage.2020.116747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman, A. I. , & Wilson, T. W. (2020). Attention modulates the gating of primary somatosensory oscillations. NeuroImage, 211, 116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Fleischer, A. , Archer, D. , Hayasaka, S. , & Sawaki, L. (2011). Oscillatory MEG motor activity reflects therapy‐related plasticity in stroke patients. Neurorehabilitation and Neural Repair, 25(2), 188–193. 10.1177/1545968310378511 [DOI] [PubMed] [Google Scholar]
- Wilson, T. W. , Heinrichs‐Graham, E. , & Becker, K. M. (2014). Circadian modulation of motor‐related beta oscillatory responses. NeuroImage, 102(2), 531–539. 10.1016/j.neuroimage.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Heinrichs‐Graham, E. , Robertson, K. R. , Sandkovsky, U. , O'Neill, J. , Knott, N. L. , Fox, H. S. , & Swindells, S. (2013). Functional brain abnormalities during finger‐tapping in HIV‐infected older adults: A magnetoencephalography study. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology, 8(4), 965–974. 10.1007/s11481-013-9477-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Lew, B. J. , Spooner, R. K. , Rezich, M. T. , & Wiesman, A. I. (2019). Aberrant brain dynamics in neuroHIV: Evidence from magnetoencephalographic (MEG) imaging. Progress in Molecular Biology and Translational Science, 165, 285–320. 10.1016/bs.pmbts.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Slason, E. , Asherin, R. , Kronberg, E. , Reite, M. L. , Teale, P. D. , & Rojas, D. C. (2010). An extended motor network generates beta and gamma oscillatory perturbations during development. Brain and Cognition, 73(2), 75–84. 10.1016/j.bandc.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Slason, E. , Asherin, R. , Kronberg, E. , Teale, P. D. , Reite, M. L. , & Rojas, D. C. (2011). Abnormal gamma and beta MEG activity during finger movements in early onset psychosis. Developmental Neuropsychology, 36(5), 596–613. 10.1080/87565641.2011.555573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley, K. J. , Andermann, M. , Koulis, T. , MacDonald, D. , & Evans, A. C. (1999). Detecting changes in nonisotropic images. Human Brain Mapping, 8(2–3), 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley, K. J. , Marrett, S. , Neelin, P. , Vandal, A. C. , Friston, K. J. , & Evans, A. C. (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping, 4(1), 58–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this article will be made publicly available through the COINS framework at the completion of the study (https://coins.trendscenter.org/).


