Fig. 2.
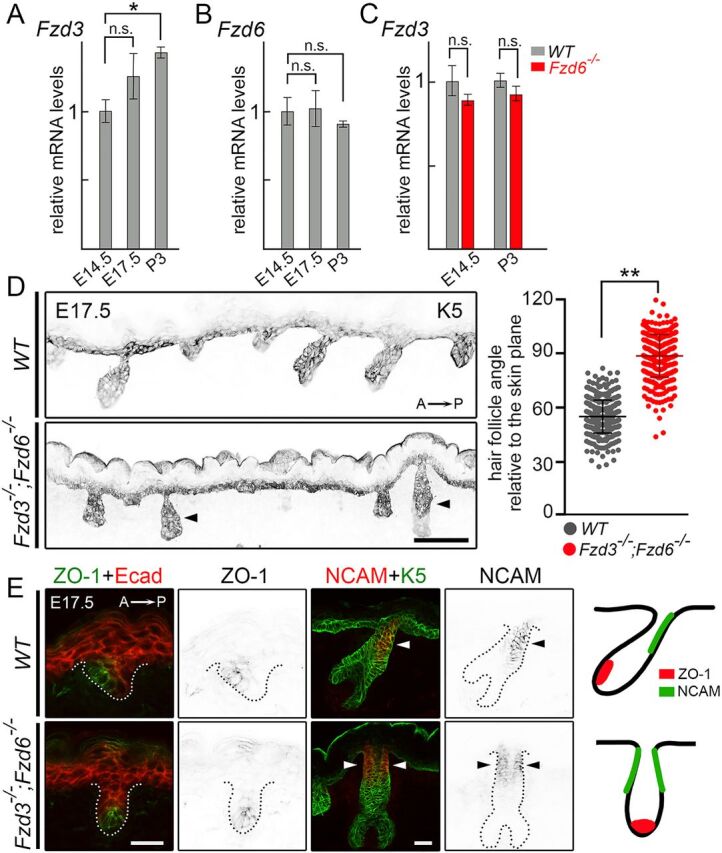
Fzd3 and Fzd6 act redundantly in controlling hair follicle polarity. (A) Expression of Fzd3 in WT skins by qRT-PCR. (B) Expression of Fzd6 in WT skins by qRT- PCR. (C) Expression of Fzd3 in WT and Fzd6−/− skin by qRT-PCR. All data are mean±s.e.m. of three biological replicates. GAPDH was used as a control. In A and B, the mRNA expression levels of Fzd3 and Fzd6 in E17.5 and P3 back skins were compared with E14.5 skins using ANOVA followed by Dunnett's multiple comparison test. In C, the Fzd3 expression levels in WT and Fzd6 knockout skins were compared using the Student's t-test at both time points (*P<0.05; n.s., not significant). (D) Epithelial cells and developing hair follicles were visualized by keratin 5 (K5) immunostaining on sagittal sections of E17.5 back skins. In the absence of both Fzd3 and Fzd6, hair follicles are vertically oriented. Hair follicle angles to the plane of the skin were compared using the Student's t-test. **P<0.01. WT, n=456 hair follicles; Fzd3−/−; Fzd6−/−, n=402 hair follicles. Arrowheads indicate vertically oriented hair follicles. (E) Complete loss of anterior-posterior polarity in Fzd3−/−; Fzd6−/− hair follicles. Sagittal sections of E17.5 back skins stained with anterior marker ZO-1 (left panels) or posterior marker NCAM (middle panels). E-cadherin (Ecad) and K5 antibodies were used to highlight skin epithelia and hair follicles. Dotted lines outline the hair follicles. Diagrams show the asymmetrical distribution of ZO-1 and NCAM in WT hair follicles and the complete loss of anterior-posterior polarity in Fzd3−/−; Fzd6−/− hair follicles (right panels). Arrowheads indicate NCAM expressing cells. A→P, anterior-to-posterior. Scale bars: 100 µm in D; 25 µm in E.
