Abstract
Coordination between the endoderm and adjacent cardiac mesoderm is crucial for heart development. We previously showed that myocardial migration is promoted by convergent movement of the endoderm, which itself is controlled by the S1pr2/Gα13 signaling pathway, but it remains unclear how the movements of the two tissues is coordinated. Here, we image live and fixed embryos to follow these movements, revealing previously unappreciated details of strikingly complex and dynamic associations between the endoderm and myocardial precursors. We found that during segmentation the endoderm underwent three distinct phases of movement relative to the midline: rapid convergence, little convergence and slight expansion. During these periods, the myocardial cells exhibited different stage-dependent migratory modes: co-migration with the endoderm, movement from the dorsal to the ventral side of the endoderm (subduction) and migration independent of endoderm convergence. We also found that defects in S1pr2/Gα13-mediated endodermal convergence affected all three modes of myocardial cell migration, probably due to the disruption of fibronectin assembly around the myocardial cells and consequent disorganization of the myocardial epithelium. Moreover, we found that additional cell types within the anterior lateral plate mesoderm (ALPM) also underwent subduction, and that this movement likewise depended on endoderm convergence. Our study delineates for the first time the details of the intricate interplay between the endoderm and ALPM during embryogenesis, highlighting why endoderm movement is essential for heart development, and thus potential underpinnings of congenital heart disease.
Keywords: Myocardial migration, Endoderm convergence, Subduction, S1pr2/Gα13, In vivo imaging
Summary: During early zebrafish heart development, three distinct phases of endoderm convergence and myocardial cell migration can be identified, all of which rely on S1pr2/Gα13 signalling.
INTRODUCTION
Interplay between adjacent tissues is crucial for organ formation. During vertebrate development, the heart tube is formed by the fusion of two populations of myocardial precursors, which migrate from bilateral regions of embryos. This migration requires proper proliferation, differentiation and epithelial organization of myocardial cells, as well as an environment conducive to their migration (Bakkers, 2011; Evans et al., 2010; Staudt and Stainier, 2012).
Among the environment factors crucial for myocardial migration is the endoderm, a tissue adjacent to the cardiac mesoderm. Mouse and zebrafish mutants with endodermal defects display cardia bifida (David and Rosa, 2001; Narita et al., 1997; Reiter et al., 1999), and in chick embryos, disruption of the endoderm perturbs myocardial migration (Rosenquist, 1970). The endoderm has been reported to influence heart formation by secreting growth factors required for cardiac specification and differentiation (Andrée et al., 1998; Lough and Sugi, 2000; Nascone and Mercola, 1995; Schultheiss et al., 1995). However, this is not always the case; for example, myocardial differentiation is unaffected in zebrafish mutants that lack endoderm (Yelon et al., 1999). The endoderm is also thought to provide a physical substrate over which cardiac precursors migrate, because of the close anatomic proximity of these tissues and the finding that in most animal models, myocardial migration fails in the absence of endoderm (David and Rosa, 2001; Lough and Sugi, 2000). Recent work in chick, however, suggests that endodermal movement directs migration of the heart field by providing mechanical forces that pull cardiac mesoderm to the midline (Varner and Taber, 2012), and that active migration by the cardiac cells is minimal (Cui et al., 2009). Similarly, in mouse, invagination of the endoderm-derived foregut is directly linked to migration of the pericardial mesoderm (Madabhushi and Lacy, 2011; Maretto et al., 2008). Recently, in Drosophila the neighboring ectoderm regulates cardiac movement at certain stages (Haack et al., 2014). Thus, the movement of tissues surrounding myocardial cells can influence their migration.
In zebrafish, myocardial migration requires signaling by sphingosine-1-phosphate (S1P) via its cognate G protein-coupled receptor S1pr2. Zebrafish mutants that lack either the S1P-receptor (S1pr2; miles apart) or the S1P transporter (Spns2; two of hearts) develop cardia bifida as a result of defective myocardial migration (Kawahara et al., 2009; Kupperman et al., 2000; Osborne et al., 2008). We recently identified Gα13 as the downstream effector of S1pr2 in regulating myocardial migration, and showed that S1pr2/Gα13 signaling regulates convergent movement of the endoderm, which in turn promotes myocardial migration (Ye and Lin, 2013). However, exactly how S1pr2/Gα13-mediated endoderm movement controls myocardial migration remains unclear.
Here, we employ transgenic lines that label specifically cardiac precursors and the endoderm to assess directly developmental interactions between these tissues. Our study delineates the highly dynamic associations between the endoderm and myocardial cells, as well as other cells of mesodermal origin, during segmentation, thereby identifying a previously unappreciated movement of the mesodermal cells from the dorsal to the ventral side of the endoderm.
RESULTS
S1pr2/Gα13-mediated convergent movement of the endoderm regulates myocardial migration
To determine whether the endodermal defect is the root cause of the impaired myocardial migration observed in S1pr2/Gα13-deficient embryos, we generated a transgenic line (sox17:mCherry-2A-gna13a) in which Gα13a [a morpholino (MO)-insensitive form] is expressed specifically in the endoderm under control of the sox17 promoter (Woo et al., 2012). Embryos obtained from crossing these animals with Tg(my7:EGFP) and Tg(sox17:EGFP) fish (Mizoguchi et al., 2008) showed that the expression of mCherry (Gα13a) was detected only in the EGFP-labeled endoderm (Fig. 1A-A″). Immunofluorescence analysis revealed that in the transgenic embryos injected with gna13a/b MO, Gα13 was expressed in the endoderm but not in the adjacent mesodermal cells (supplementary material Fig. S1), confirming that transgenic endodermal Gα13a expression is MO-resistant.
Fig. 1.
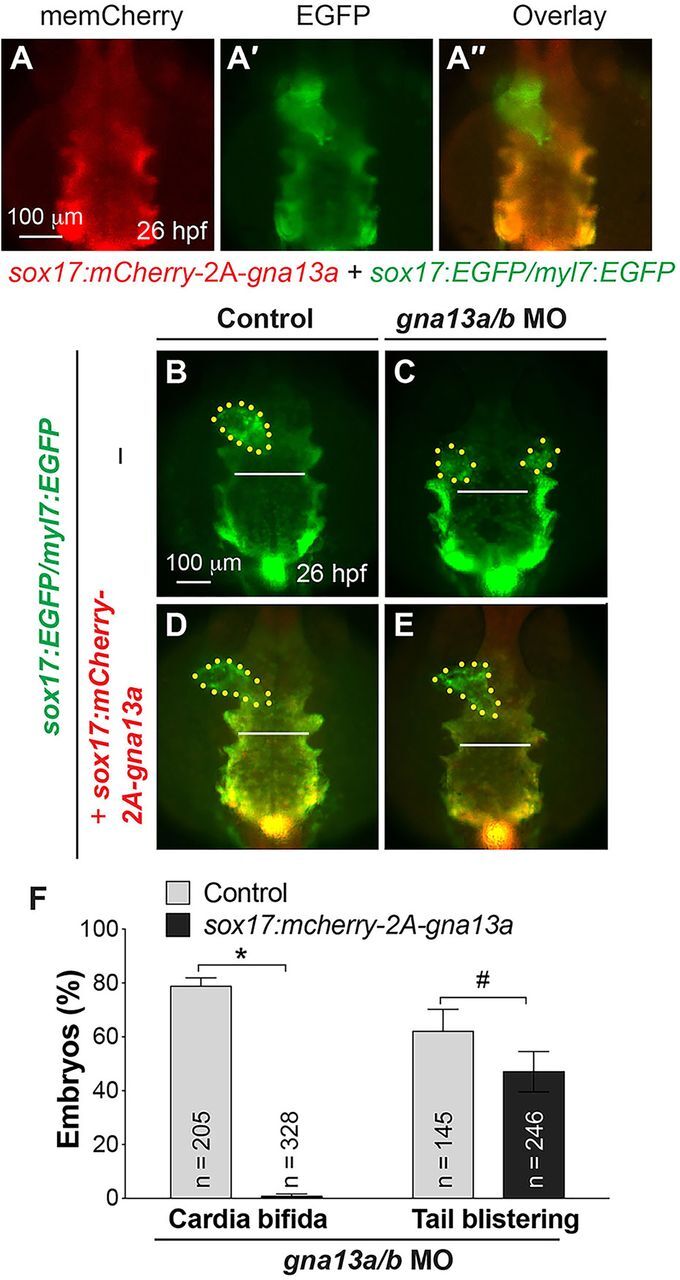
Endoderm-specific expression of Gα13 rescues defects in migration of the endoderm and myocardial cells caused by global Gα13 depletion. (A-A″) Epifluorescence images of anterior endoderm and myocardial cells in embryos indicated. (B-E) Epifluorescence images of anterior endoderm of control and gna13a/b MO-injected embryos indicated. (F) Frequencies of cardia bifida and tail blistering in embryos indicated (same as in B-E) at 2 dpf. Dorso-anterior view, with anterior up; yellow dots, cardiomyocytes; white lines (equivalent length), width of the anterior endodermal sheet. *P<0.001, #P=0.25. Data are mean±s.e.m.
Our results indicate that endodermal expression of Gα13a does not affect either endoderm convergence or myocardial migration, as the endoderm appeared to be normal and a single heart tube formed (Fig. 1D). As we reported previously (Ye and Lin, 2013), control embryos injected with gna13a/b MO (morphants) exhibited a widened endodermal sheet, cardia bifida, a lack of circulation, and pericardia edema (80%, n=205) (Fig. 1C,F; supplementary material Fig. S2B,B″; and data not shown). By contrast, Tg(sox17:mCherry-2A-gna13a) embryos injected with the MO had a single heart, normal circulation and normal endoderm morphology (99.2%, n=328; Fig. 1E,F; supplementary material Fig. S2D,D″; and data not shown), although they exhibited tail-fin blistering (47±7.5%, P=0.25; Fig. 1F; supplementary material Fig. S2D,D′), which is another characteristic phenotype in S1pr2/Gα13-deficient embryos (54±3.3%, n=205; supplementary material Fig. S2B,B′) (Kupperman et al., 2000; Ye and Lin, 2013). These findings indicate that endodermal expression of Gα13a is sufficient to rescue the endodermal defects and cardia bifida, and that the tail-fin defect is independent of cardia bifida in these animals. Thus, endoderm convergence is crucial for the migration of myocardial precursors.
The endoderm displays three distinct phases of convergent movement during segmentation
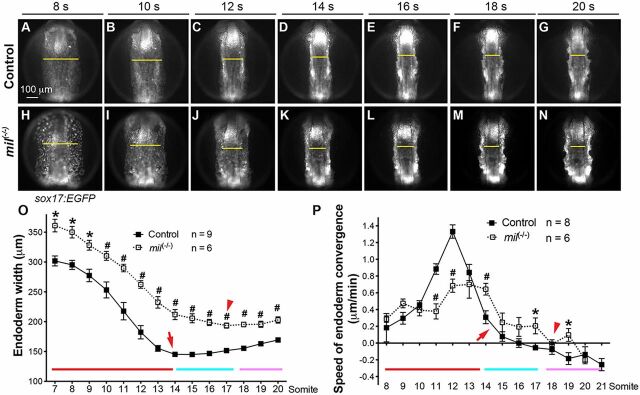
To investigate how endoderm convergence influences myocardial migration, we first examined the dynamic movements of the endoderm. We performed time-lapse experiments on the anterior region of the endoderm (in which myocardial precursors migrate) of Tg(sox17:EGFP) embryos during the 7-20 somite stage(s) (supplementary material Movie 1), as previously reported (Ye and Lin, 2013). We found that the endoderm underwent three distinct phases of movement. First, at 7-14 s, the endoderm converged rapidly towards the midline, reaching its narrowest point by 14 s, with a significant reduction (49%) in endoderm width (from 308 to 157 µm; Fig. 2A-D,O). Second, at 14-17 s, the convergence slowed to a minimum, producing no significant change in endoderm width (from 148 to 150 µm; Fig. 2D,E,O). Third, at 18-20 s, the endoderm expanded slightly, increasing its width (from 150 to 164 µm; Fig. 2F,G,O). Similarly, we determined the speed of convergence by analyzing the displacement of the lateral limits of the endoderm at various developmental stages, and found that it accelerated from 0.2 to 0.5 µm/min at 8-10 s, peaked at 1.2 µm/min at 12 s, and then dramatically fell to 0.2 µm/min by 14 s (Fig. 2P); it averaged 0.01 and −0.13 µm/min during 14-17 s and 18-20 s, respectively, which is consistent with the small change and the slight expansion of the endodermal sheet during these two periods (Fig. 2O,P).
Fig. 2.
S1pr2 is required for efficient endoderm convergence during segmentation. Epifluorescence time-lapse experiments were performed on control and mil mutant embryos (supplementary material Movie 1). (A-N) Snapshots of the anterior endoderm from the movies, at the stages indicated. Dorso-anterior views; yellow lines (equivalent length for embryos at the same stage), width of the anterior endodermal sheet, showing that the endodermal sheet was wider in mutant. (O,P) The average endoderm width (O) and convergence speed (P). Red, cyan and pink lines represent the periods of rapid convergence, little convergence and expansion, respectively. Arrows and arrowheads mark completion of convergence. *P<0.05; #P<0.01 versus control. Data are mean±s.e.m.
In s1pr2/mil mutants, at 8 s, the endodermal sheet was already significantly wider than that in the control siblings (Fig. 2H versus A), suggesting that endoderm convergence was already impaired by this stage. In these embryos, the endodermal sheet remained wider throughout segmentation, yet displayed convergence patterns similar to those of their controls (Fig. 2A-O). Notably, in these embryos, at 8-10 s the endoderm converged at speeds comparable to those in controls; however, speed acceleration did not occur until 12 s and the peak speed was slower (0.68 versus 0.98 µm/min in controls) (Fig. 2P). Thus, the reduction in endodermal width was significantly smaller than that in controls (110 versus 151 µm). Furthermore, at 15-17 s the endoderm continued to converge at a speed of 0.21 µm/min, whereas the control endoderm had completed convergence by 14 s (Fig. 2O,P). During 18-20 s, the speed of expansion was also reduced relative to that in controls (−0.04 versus −0.16 µm/min; Fig. 2P). Thus, in control embryos, endoderm convergence occurs mainly before 14 s and is rapid from 10-12 s; however, in s1pr2/mil mutant embryos, the acceleration is delayed and inefficient (Fig. 2P). The defects in rapid convergence in s1pr2/mil mutants probably contribute to the widening of the endodermal sheet throughout segmentation. Similar results were found in gna13a/b morphants (supplementary material Fig. S3). Overall, these data demonstrate that S1pr2/Gα13 signaling is required for efficient endoderm convergence during segmentation, a period during which myocardial migration occurs.
Myocardial cells associate with the endoderm during its three phases of convergence
Our observations indicate that the endoderm completes convergence at 14 s (Fig. 2), which is earlier than when myocardial precursors have been reported to migrate to the midline (16-20 s) (Holtzman et al., 2007). Moreover, our data indicate that the endoderm expands slightly from 18-20 s (Fig. 2). Thus, it seems that endoderm convergence and myocardial migration do not occur during the same time window. To understand how endoderm convergence affects myocardial migration, we characterized interactions between the two cell populations during heart-tube formation.
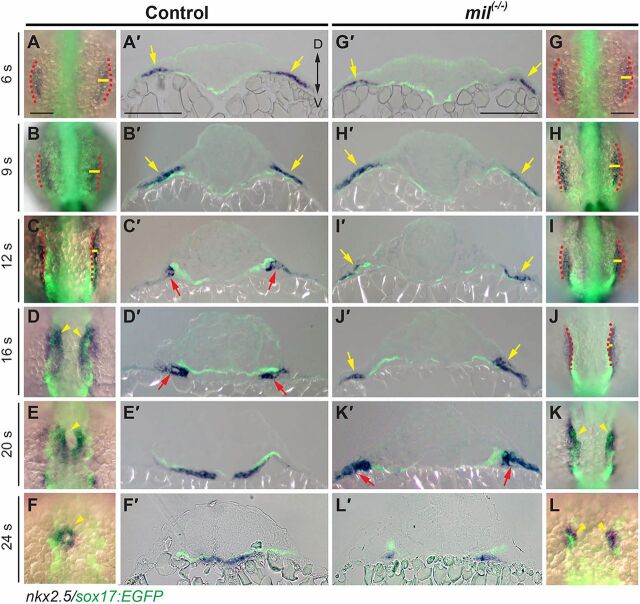
In a first approach, we evaluated the relative positions of the endoderm and myocardial precursors in fixed Tg(sox17:EGFP) embryos at various developmental stages. We performed in situ hybridization (ISH) using a probe for the cardiac transcription factor nkx2.5, labeling myocardial precursors, and then conducted GFP immunostaining to assess endoderm morphology. Our results revealed intricate and dynamic changes in the anatomy of the two tissues (Fig. 3). Before 12 s, the anterior endoderm narrowed, as shown in Fig. 2, and the myocardial precursors migrated towards the midline (the distance between the two populations became smaller) (Fig. 3A-C); notably, migrating myocardial precursors populated, and remained at, the lateral boundary of the endodermal sheet (Fig. 3A,B). These data indicate that myocardial precursors and the endodermal layer co-migrated and retained their relative positions. At 12 s, however, the myocardial precursors were located outside the lateral boundary of the endoderm (Fig. 3C), suggesting that the endoderm was uncoupled from myocardial cells and had moved further than the myocardial cells. During 16-24 s, endoderm width changed very little, but two myocardial populations moved closer to the midline and merged to form the heart cone (Fig. 3D-F), suggesting that these two tissues migrate independently during this period.
Fig. 3.
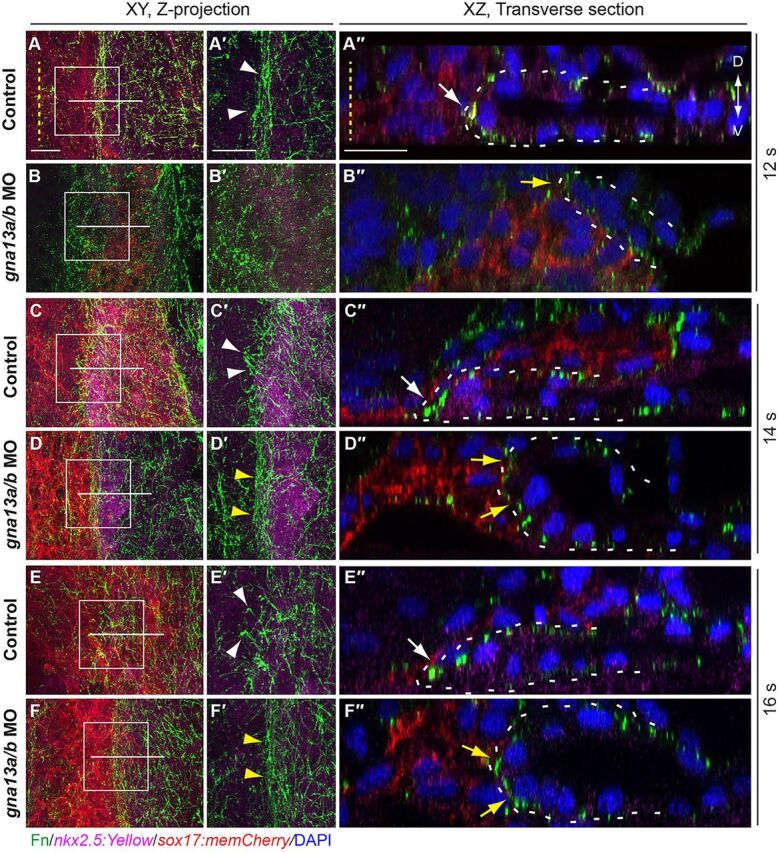
S1pr2 is required for the subduction and active migration of myocardial precursors. (A-L) Overlays of GFP-labeled endoderm (as revealed by immunofluorescence) and nxk2.5 expression (as revealed by whole-mount in situ hybridization), in embryos at the stages indicated. (A′-L′) Transverse sections of the embryos shown in A-L. Red dashed lines indicate lateral boundaries of the endodermal sheet; yellow lines denote width of the area covered by the myocardial populations; arrowheads indicate myocardial cells; arrows indicate that myocardial cells are located above (yellow) and below (red) the endoderm. Scale bars: 100 µm.
Additionally, we observed that the morphology of the myocardial population changed during this period: before 12 s, the cell clusters had a broad spindle-like shape (Fig. 3A,B); at 12 s, they became more compact and condensed into narrow strips (Fig. 3C); by 16 s, they were significantly expanded and had migrated towards each other (Fig. 3D); and by 24 s the clusters had formed a heart cone (Fig. 3E,F). Transverse sections of these embryos revealed an intriguing and unexpected phenomenon (Fig. 3A′-F′): before 9 s, the myocardial precursors laid dorsal to the endoderm; by 12 s, they had moved ventral to the endoderm, and this migration was similar to the subduction that had been described in Caudata and Xenopus, i.e. the movement of cells from a superficial to a deeper location (Shook et al., 2002, 2004); by 16 s, the myocardial precursors were all ventral to the endoderm.
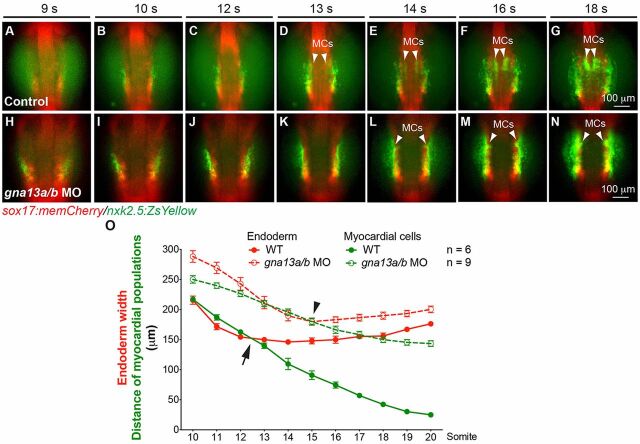
In a second approach, we employed in vivo imaging to follow the migration of the endoderm and myocardial precursors. We utilized Tg(nkx2.5:ZsYellow) (Zhou et al., 2011), in which ZsYellow can be detected in myocardial precursors as early as 9-10 s, and our newly generated line Tg(sox17:memCherry), which expresses membrane-localized mCherry in the endoderm (Fig. 4A). Consistent with our findings in fixed embryos (Fig. 3), during 9-12 s the ZsYellow-expressing myocardial populations were located at the lateral boundaries of the endoderm, and migrated with the endoderm towards the midline (Fig. 4A-C; supplementary material Movie 2). Further measurement showed that the width of the endoderm and the distance between two myocardial populations were very similar (Fig. 4O), confirming that the endoderm and myocardial cells migrated together and at similar speed. However, at 12-13 s these tissues became uncoupled (Fig. 4O, arrow), with the endoderm moving minimally but myocardial precursors continuing their migration towards the midline until they merged and formed the heart cone (Fig. 4D-G,O). At 17-20 s, in vivo imaging on Tg(nkx2.5:lifeact-GFP) embryos revealed that myocardial cells at the leading region extended active protrusions towards the midline (supplementary material Movie 3 and Fig. S4C-F).
Fig. 4.
Gα13 is required for all stages of myocardial migration. Epifluorescence time-lapse experiments performed on the embryos indicated (supplementary material Movie 2). (A-N) Snapshots of the anterior endoderm and myocardial cells from the movies at the stages indicated. Dorso-anterior views. White arrowheads indicate myocardial cells (MCs). (O) Endoderm width (red) and the distance between the two populations of myocardial cells (green) at the stages indicated. Black arrow and arrowhead denote the timepoints at which myocardial precursors were dissociated from the endoderm in the control and gna13a/b MO-injected embryos, respectively. Data are mean±s.e.m.
Collectively, these data indicate that myocardial precursors undergo migration in three major stages that might involve different mechanisms (Fig. 6E, model): (1) before 12 s, they depend on the endoderm to migrate towards the midline; (2) at 12-14 s, they undergo subduction, moving from the dorsal to the ventral side of the endoderm; and (3) after 14 s, they engage in migration towards the midline, independent of convergent movement of the endoderm.
Fig. 6.
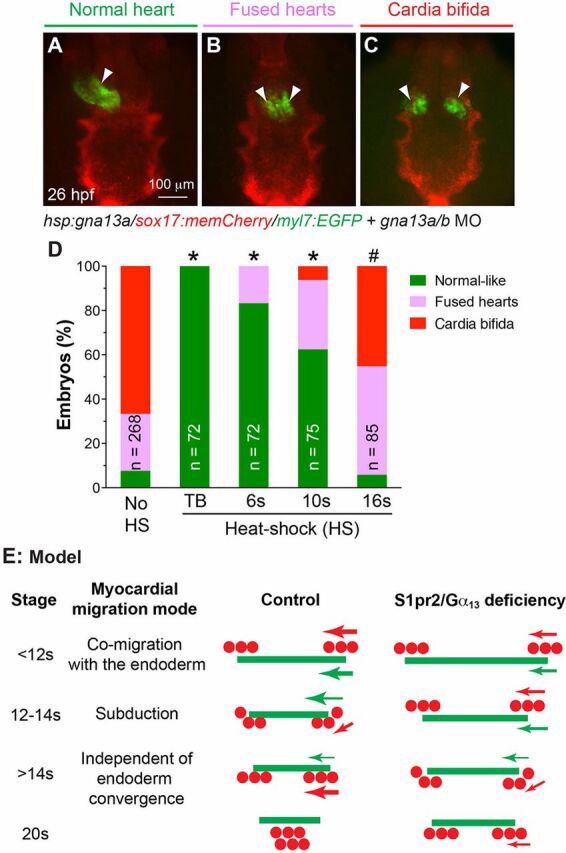
Gα13 expression prior to myocardial subduction is crucial for myocardial migration. (A-C) Classes of heart morphology phenotypes at 26 hpf, in embryos heat shocked at various stages: normal heart (A), two fused hearts (B) and two separated hearts (C). Arrowheads denote hearts. (D) Distribution of heart phenotype classes in embryos heat shocked at the indicated stages of development. The total number of embryos is indicated. *P<0.001; #P=0.8, percentage of normal-like embryos in indicated groups versus non-heat shocked group. (E) Model of how the endoderm and myocardial cells interact during heart-tube formation. The relative positions and velocities of migration of the endodermal sheet (green) and myocardial precursors (red dots) in control and S1pr2/Gα13-defective embryos at the indicated stages of myocardial-cell migration, with the mode of migration indicated. Arrow direction denotes direction of migration; arrow thickness indicates speed of migration.
S1pr2/Gα13 signaling is required for all stages of myocardial migration
Next, we investigated which stage(s) of myocardial migration is affected in S1pr2/Gα13-deficient embryos. We found that in mil mutant embryos, myocardial precursors populated at the lateral boundaries of the widened anterior endodermal sheet as in their control counterparts, but that they remained in these positions for a much longer period (6-16 s versus 6-12 s) (Fig. 3G-J versus A-C). Similar results were found in gna13a/b morphants using transgenic lines in which the myocardial and endodermal cells are labeled (supplementary material Fig. S3F-I versus A,B). Live imaging revealed that myocardial precursors and the endodermal layer remained at constant relative positions and migrated together for a much longer period, until 16 s (Fig. 4H-M versus A-C,O; supplementary material Movie 2). These findings indicate that in S1pr2/Gα13-deficient embryos, the first phase of myocardial migration was impaired because of the defect in endoderm convergence. Furthermore, in embryos globally deficient for Gα13, endodermal expression of Gα13 rescued defects in both endoderm convergence and myocardial migration (supplementary material Movie 4). These data support our hypothesis that, prior to subduction, myocardial precursors depend on the endoderm to migrate.
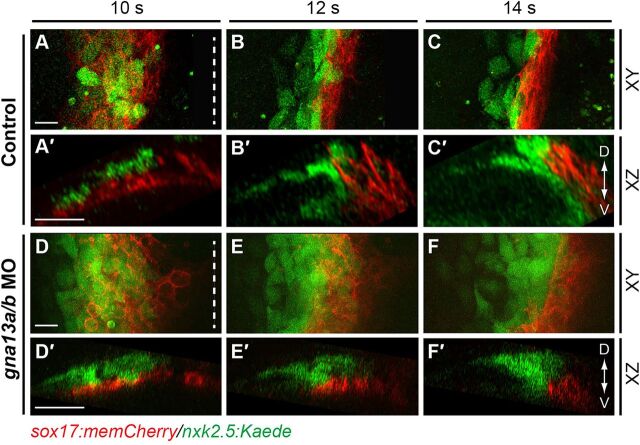
When myocardial cells engaged in subduction at ∼12 s, the shape of the myocardial population changed from spindle- to stripe-like (Fig. 3B versus C), and the cells migrated from the dorsal to the ventral side of the endoderm (Fig. 3B′ versus C′). Thus, this stripe reflects a morphological change in the myocardial population during subduction. Notably, in mil mutant embryos and gna13a/b morphants, appearance of the stripe was delayed until around 16 s (Fig. 3J; supplementary material Fig. S3I), with myocardial cells first emerging on the ventral side of the endoderm at this time (Fig. 3J′; supplementary material Fig. S3I′). To monitor subduction, we performed confocal in toto imaging on Tg(sox17:memCherry)/Tg(nkx2.5:Kaede) embryos (Fig. 5). We analyzed the XZ views to determine the spatial organization of memCherry-expressing endoderm and Kaede-expressing myocardial cells. Consistent with the findings in Fig. 3, in control embryos myocardial precursors located dorsal to the endodermal layer at 10 s (Fig. 5A′), but as the endodermal sheet converged at 12-14 s, they slid underneath the endoderm (Fig. 5B′-C′), indicating that myocardial subduction is a relatively quick process, lasting about 30 min (11-12 s, Fig. 5A′-B′; supplementary material Movie 5). An earlier study had shown that myocardial populations displayed two-layered structures from 16 s (Trinh and Stainier, 2004). Here, we found that the myocardial populations appeared to be a single monolayer before subduction, and were converted to two layers during the subduction period (Fig. 5A′ versus B′-C′). Notably, cell tracking revealed that the lateralmost cells (3 embryos, 3-5 cells per embryo) migrated towards the midline (a phenomenon that could contribute to formation of a second layer of the myocardial cells), whereas the dorsalmost cells stayed in the same relative position (supplementary material Movie 5, control, cell #1 versus cell #2). By contrast, in S1pr2/Gα13-deficient embryos, subduction of the myocardial cells initiated at 13-14 s (Fig. 5E-F′) and lasted more than 60 min (13-16 s; Fig. 3I-L, Fig. 5F′; supplementary material Fig. S3H-I″, Movie 5). Thus, subduction of myocardial precursors occurred in the context of the S1pr2/Gα13 deficiency but was significantly delayed and inefficient.
Fig. 5.
Subduction of myocardial precursors is impaired in Gα13 morphants. (A-F′) Snapshots from in toto confocal time-lapse movies of control (A-C′) or gna13a/b MO-injected (D-F′) Tg(sox17:memCherry)/(nkx2.5:Kaede) embryos at 10-14 s (supplementary material Movie 5). XY view (A-F), XZ view (A′-F′). Dashed lines denote the midline. D, dorsal; V, ventral. Scale bars: 20 µm.
Finally, in control embryos, after subduction (12 s) the myocardial cells were positioned ventral to the endoderm, and from 18-20 s they migrated towards the embryonic midline to form the heart cone (Fig. 3D,E and Fig. 4E-G). In S1pr2/Gα13-deficient embryos, the myocardial cells exhibited little medial migration, even after they had moved beneath the endoderm (Fig. 3K,L, Fig. 4M,N; supplementary material Fig. S3J, Movie 2); consistent with this finding, these cells formed protrusions only briefly (supplementary material Fig. S4G-K). These data indicate that S1pr2/Gα13 signaling is required for the migration of myocardial precursors at all stages.
Endoderm convergence controls the initial migration and subduction of myocardial cells
The observation that myocardial migration (during 17-20 s) is impaired in the context of the S1pr2/Gα13 deficiency seems to contradict the findings that myocardial migration is independent of endoderm convergence at this stage (Figs 3 and 4). To identify precisely which stage(s) of myocardial migration requires S1pr2/Gα13-mediated endoderm convergence, we manipulated Gα13a expression temporally by utilizing a transgenic Tg(hsp70:gna13a) line that expresses Gα13a under the control of the heat shock promoter hsp70 (Kwan et al., 2007). Embryos derived from crosses between Tg(hsp70:gna13a) and Tg(sox17:memCherry) animals were injected with the gna13a/b MO.
At the following stages (during which myocardial precursors undergo different modes of migration), the injected embryos were placed at 37°C for 30 min to induce the expression of Gα13a: the tail-bud (TB) stage, 6 s, 10 s and 16 s. The expression of targeted proteins can be detected after 45 min of heat shock treatment (Shoji and Sato-Maeda, 2008). Thus, heat shock (HS) at the TB stage is expected to cause Gα13 expression at 2-3 s, before myocardial subduction is initiated; HS at 6 s will induce Gα13 expression at 8-9 s, just before myocardial subduction occurs; HS at 10 s and 16 s will induce Gα13 expression at 12-13 s and 18-19 s, when myocardial cells undergo and complete subduction, respectively. Indeed, immunostaining assay revealed that in control embryos Gα13 expression was present in both endodermal and non-endodermal cells (supplementary material Fig. S5A,B), but in gna13a/b morphants it was present at 13 s only if HS was applied at TB (supplementary material Fig. S5C versus D). Both control and HS embryos were evaluated for heart morphology at 26 hpf, by which time control myocardial cells would have fused and formed a single heart (Fig. 6A), and embryos with defects in myocardial migration typically have two hearts, which can either be separated completely (cardia bifida, Fig. 6C) or fused (Fig. 6B).
We found that Gα13 expression at different stages rescued myocardial migration defects to varying degrees. Consistent with our previous findings (Ye and Lin, 2013), among morphants that were not subjected to HS, 64.4±7.8% displayed cardia bifida, 27.9±5.9% exhibited fused hearts and only 7.7±3.3% had a single heart (Fig. 6D). Strikingly, when subjected to HS at the TB stage, 100% morphants had a single heart (Fig. 6D, P=5.3E-07 versus non-HS), suggesting that myocardial migration defects in gna13a/b morphants were completely rescued by Gα13 expression at this stage. When subjected to HS at 6 s, 16.8±12.8% of morphants had fused hearts, and the rest (83.2±12.8%, P=7E-06 versus non-HS) had single hearts, suggesting that Gα13 expression just prior to myocardial subduction rescues myocardial migration significantly, although not fully. HS at 10 s (induces Gα13 expression at 12-13 s, prior to or during myocardial subduction in gna13a/b morphants) led to 6.9±4.3% of embryos exhibiting cardia bifida, 33.1±3.8% fused hearts and 65±3.4% (P=3.3E-6 versus non-HS) normal hearts (Fig. 6D), suggesting that Gα13 expression during myocardial subduction rescued the myocardial migration defects only partially. Finally, in embryos subjected to HS at 16 s, when myocardial subduction is complete, the incidence of fused hearts and cardia bifida was similar to that of non-HS controls (94.2% versus 92.3%, respectively; P=0.8), although they more often displayed fused hearts (48.9±5.2% versus 27.9±5.9% in non-HS control) than cardia bifida (45.3±4.7% versus 64.4±7.8% in non-HS controls) (Fig. 6D). This suggests that the induction of Gα13 expression at 16 s has only a minor effect on myocardial migration.
Overall, our rescue experiments indicate that: (1) the earlier in segmentation Gα13 is expressed in the context of a Gα13 deficiency, the better the chances that defects in myocardial migration will be rescued; (2) endodermal Gα13 expression (and thus normal endoderm convergence) at 12-14 s is essential for myocardial migration and subduction; and (3) Gα13 expression after myocardial subduction is insufficient to rescue defects in myocardial migration (Fig. 6E, model).
Organization of the myocardial epithelium is disrupted in Gα13 morphants
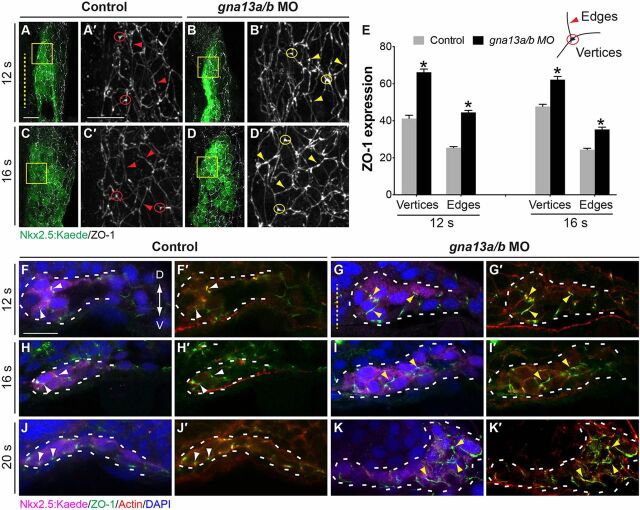
The epithelial organization of myocardial cells matures from 16-20 s, and this step is crucial for their migration (Garavito-Aguilar et al., 2010; Trinh and Stainier, 2004). To explore the cellular mechanisms that underlie myocardial defects in the context of the Gα13 deficiency, we first assessed expression of the polarity marker ZO-1 in myocardial cells. First, we performed whole-mount immunostaining in Tg(nkx2.5:Kaede) embryos at 12 s and 16 s, the times at which myocardial cells undergo and complete subduction, respectively, and analyzed Z-projections of XY views of Kaede-labeled cells (Fig. 7A-E). In control myocardial cells, ZO-1 expression was observed at the cell edges, with enrichment at the vertices (where three or more cells meet; Fig. 7A′,C′, circles), and faint punctate structures were presented in some areas (Fig. 7A′,C′, arrowheads). In Gα13-deficient myocardial cells, the punctate ZO-1 staining at the cell edges was much more prominent (Fig. 7B′,D′, arrowheads), and the ZO-1 expression was significantly increased at both the edges and vertices (Fig. 7A-E, circles and arrowheads).
Fig. 7.
Epithelial organization of myocardial cells is disrupted in Gα13 morphants. (A-E) Whole-mount ZO-1 immunostaining was performed in embryos indicated at 12 s and 16 s. (A-D) Projections of confocal z-stacks of the right lateral mesoderm showing ZO-1 expression (gray) in the nkx2.5:Kaede-expressing (myocardial) cells (green). (A′-D′) Higher-magnification views of areas shown in boxes in A-D. The vertices were defined as regions where membranes from three adjacent cells come into contact, and the edges as the cell periphery excluding the vertices. Yellow dashed line, midline; circles, vertices; arrowheads, edges. (E) Intensity of ZO-1 expression in myocardial cells at the vertices and edges in control embryos (54 cells from 7 embryos at 12 s and 25 cells from 4 embryos at 16 s) and gna13a/b MO-injected embryos (51 cells from 8 embryos at 12 s and 20 cells from 4 embryos). *P<0.001 versus control. Data are mean±s.e.m. (F-K) Transverse vibratome sections immunostained for Kaede (magenta), ZO-1 (green), actin (Rhodamine-Phalloidin, red) and nuclei (DAPI, blue). Yellow dashed line indicates midline; white dashed lines outline myocardial cells; white and yellow arrowheads indicate normal and ectopic ZO-1 expression, respectively. Scale bars: 20 µm.
To examine the epithelial organization of myocardial precursors in greater depth, we performed immunostaining on vibratome-sectioned embryos. In control myocardial cells, punctate ZO-1 expression was present in the lateral membrane between cells starting at 12 s, and became restricted to the basal domain of the lateral membrane by 20 s (Fig. 7F,H,J, white arrowheads), indicating that the epithelial organization matured during myocardial migration, consistent with a previous report (Trinh and Stainier, 2004). Notably, the timing of ZO-1 expression at 12 s (Fig. 7F,F′) coincided with that of the initiation of subduction, suggesting that in addition to contributing to myocardial migration at later stages, ZO-1 expression might be important for subduction. Consistent with our findings from Z-projections, in Gα13-deficient embryos ZO-1 expression was diffuse and its domain was expanded, with some localized in ectopic regions such as the apical membrane (Fig. 7G,I,K, yellow arrowheads). In addition, two other polarity markers, αPKC and β-catenin, are localized apicolaterally and basolaterally, respectively, in control myocardial cells (Trinh and Stainier, 2004). In the mil mutant and Gα13 morphants, these proteins were mislocalized, concomitant with the aberrant ZO-1 expression (supplementary material Fig. S6). Collectively, our findings indicate that in S1pr2/Gα13-deficient embryos the epithelial organization of the myocardial population is disrupted – a feature that might lead to impairment of their subduction and their medial migration at later stages.
Fibronectin (Fn) assembly surrounding myocardial cells is disrupted in Gα13 morphants
Proper expression of Fn in the microenvironment is crucial for establishing the apicobasal organization that myocardial cells need for their medial migration (Arrington and Yost, 2009; Garavito-Aguilar et al., 2010; Sakaguchi et al., 2006; Trinh and Stainier, 2004; Trinh et al., 2005). Furthermore, S1pr2/Mil has been shown to interact genetically with Fn to promote myocardial migration (Matsui et al., 2007). Similarly, we found that Gα13 interacted genetically with Fn in regulating endoderm convergence and myocardial migration (not shown). To determine whether Gα13 affects Fn expression, we examined its patterns in whole-mount embryos at various stages.
In control embryos, Fn fibrils were assembled in striking patterns that were previously unappreciated. At 12 s, when myocardial cells underwent subduction, Fn fibrils were enriched between the endoderm and the myocardial populations, and were aligned along the anterior-posterior axis (Fig. 8A,A′, white arrowheads); at 14 and 16 s, during which myocardial cells complete subduction and migrate towards the midline, the direction of Fn fibril assembly was re-aligned in the putative direction of migration of the myocardial cells (Fig. 8C,C′,E,E′, white arrowheads). Furthermore, transverse XZ sections revealed that Fn fibrils surrounded the myocardial population and were highly enriched at the leading edge of the myocardial population during subduction and medial migration (Fig. 8A″,C″,E″, white arrows). These findings suggest that the assembly of Fn fibrils is crucial for the subduction and medial migration of myocardial cells.
Fig. 8.
Fn assembly patterns are disrupted in Gα13 morphants. Whole-mount Fn immunostaining was performed in the embryos indicated. (A-F) Projections of XY views of confocal z-stacks spanning the myocardial cells (magenta), showing Fn assembly (green). (A′-F′) Magnification images of areas shown in boxes in A-F. White and yellow arrowheads indicate leading regions of myocardial populations in control and gna13a/b MO-injected embryos, respectively. (A″-F″) Images of XZ transverse sections of the regions indicated by white lines in A-F. White and yellow arrows indicate Fn assembly in the leading front of myocardial cells in control and gna13a/b MO-injected embryos, respectively. Yellow dashed line, midline; white dashed lines outline myocardial cells; D, dorsal; V, ventral. Scale bars: 20 µm.
In Gα13 morphants, overall Fn expression level is not significantly different (Fig. 8). However, Fn assembly exhibited completely different patterns: at 12 s, myocardial cells had not initiated subduction, and Fn fibrils appeared to be smaller and displayed no obvious directionality, although Fn assemblies surrounded the myocardial cells (Fig. 8B-B″); at 14 and 16 s, Fn fibrils were aligned along the anterior-posterior axis, and condensed in the lateral region of the myocardial population (Fig. 8D′,F′, yellow arrowheads); cross-sectional views revealed that Fn fibrils were not enriched, but rather expressed broadly, in the leading edge of the myocardial population (Fig. 8D″,F″, yellow arrows). Considering the crucial role that Fn assembly plays in epithelial organization of the myocardial cells, we postulate that abnormal Fn assembly might contribute to defects in their epithelial polarity, and thus to defects in their subduction and medial migration. On the other hand, the disrupted patterns of Fn assembly might reflect impairment of myocardial subduction and migration.
Cells in the ALPM engage in subduction that depends on endoderm convergence
The myocardial cells are components of the ALPM, and we tested whether other cells within this structure engage in similar subduction. The basic helix-loop-helix (bHLH) family member hand2 is expressed in the ALPM (Trinh et al., 2005; Yelon et al., 2000), and we used Tg(hand2:GFP)/Tg(sox17:memCherry) transgenic lines to monitor movement of the ALPM and endoderm. We found that two GFP+ ALPM populations, including myocardial cells (Fig. 9), flanked the endodermal layer at 10 s, at which point GFP can be detected (Fig. 9A, yellow arrowheads), and then migrated medially and merged at the midline to form a single sheet (Fig. 9A-F, supplementary material Movie 6). As shown in Fig. 3, during subduction the myocardial population formed a stripe at 10-12 s. Notably, cells other than myocardial cells located in the dorsalmost regions of the GFP+ domain exhibited a similar morphology (Fig. 9A,B, red arrowheads), suggesting that these ALPM cells also underwent subduction. Indeed, transverse sections of three-dimensional (3D) confocal z-stacks revealed that, like myocardial cells, the Hand2-GFP+ cells were dorsal to the endodermal layer at 8 s, but gradually slid underneath and were ventral to the endoderm at 10 s and 14 s (supplementary material Fig. S7A-C′).
Fig. 9.
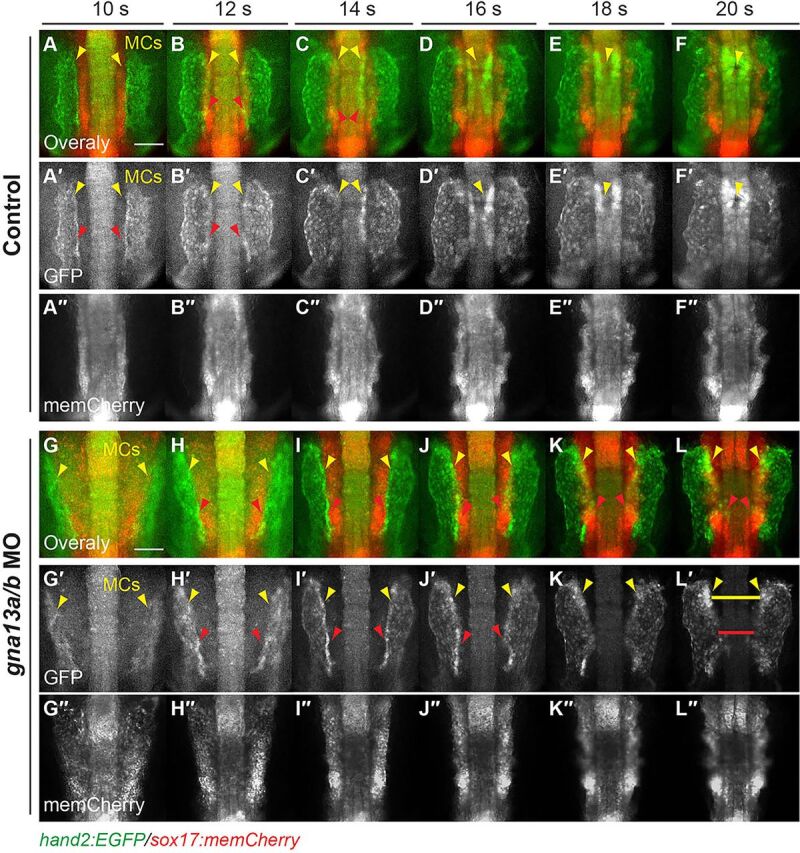
The ALPM engages in endoderm-dependent subduction. Snapshots from epifluorescence time-lapse movies of control and gna13a/b MO-injected Tg(sox17:memCherry)/(hand2:EGFP) embryos at 10-20 s (supplementary material Movie 6). Dorso-anterior views. (A-L) Overlays of anterior memCherry-labeled endoderm (red) and Hand2:GFP-labeled ALPM (green). (A′-L′) Hand2:GFP-labeled ALPM. (A″-L″) memCherry-labeled endoderm. Yellow arrowheads indicate myocardial cells (MCs); red arrowheads indicate dorsalmost region of LPM cells; yellow and red lines indicate gap between the two myocardial populations and the dorsalmost region of LPM cells. Scale bars: 100 µm.
In Gα13-deficient embryos, the ALPM cells flanked the widened endodermal layer, and those in the dorsalmost regions, including the myocardial cells, failed to migrate to the midline by 22 s, leaving a gap (Fig. 9L, yellow and red lines; supplementary material Fig. S7E-E″, Movie 6). Moreover, ALPM cells other than the myocardial cells formed a stripe at 14-16 s, and these cells did not slide underneath the endoderm until 16 s (Fig. 9H-J; data not shown), indicating that subduction of these cells was delayed and impaired. Thus, in the dorsalmost regions of Gα13-deficient embryos, all ALPM cells are impaired with respect to both subduction and medial migration, suggesting that medial migration of this tissue depends on proper convergence of the endoderm.
To test further whether the migration of ALPM cells relies on endoderm convergence, we injected a MO targeting sox32, a transcription factor required for endoderm development (Wong et al., 2012; Yelon et al., 1999). In such morphants, which lack endoderm, the Kaede-expressing myocardial precursors failed to migrate towards the midline, remaining in the same position from 12-18 s (supplementary material Fig. S7I-K versus F-H, Movie 7). In addition, unlike in control embryos, myocardial cells in the morphants failed to form the stripe that is characteristic of myocardial subduction (supplementary material Fig. S7I versus F). These findings indicate that the endoderm is crucial for the subduction and medial migration of ALPM cells.
DISCUSSION
Although the endoderm is crucial for myocardial migration, it is unclear precisely how these two tissues interact. The current study extends our previous findings that endoderm convergence is directly linked to myocardial migration. Employing newly developed transgenic lines, we have identified previously unknown features of the movements of these tissues, as well as their intricate and dynamic anatomic relationship during segmentation (Fig. 6E, model). We show that the endoderm undergoes three distinct phases of movement: rapid convergence before 12 s, little convergence at 12-16 s and slight expansion after 16 s; and that myocardial cells concomitantly undergo three distinct modes of migration: initial migration that depends on endoderm convergence (before 12 s), subduction from the dorsal to the ventral side of the endoderm (12-14 s) and medial migration that appears to be independent of endoderm convergence (after 14 s). These findings will probably be fundamental to comprehending the role of the endoderm in cardiac morphogenesis.
Myocardial cells depend on the endoderm for their migration at early stages
The dependence of myocardial migration on the converging endoderm was revealed by our imaging of live and fixed embryos, which showed that before 12 s, when the endoderm actively converges towards the midline and its width is reduced, two populations of myocardial cells flank and remain at the lateral boundaries of the endodermal sheet. Thus, at this stage myocardial precursors depend on the endoderm and co-migrate with it towards the midline (Figs 3,4; supplementary material Fig. S3, Movie 2). This notion is supported by the findings that in the context of S1pr2/Gα13 deficiency, myocardial precursors remained at the lateral boundary of the widened endodermal sheet; thus, migration of myocardial cells during this period was impaired because of defects in endoderm convergence (Figs 3,4; supplementary material Fig. S3, Movie 2). Moreover, endodermal expression of Gα13 before 12 s almost fully rescued the defects of endoderm convergence and myocardial migration caused by global inhibition of Gα13, whereas such expression after 12 s had less effect on either endoderm convergence or myocardial migration (Fig. 6; supplementary material Movie 4). Our data provide direct evidence that prior to 12 s myocardial cells rely on endoderm convergence to move to the midline. This finding is consistent with observations in other animal model systems, showing that the migration of myocardial cells is influenced by the movements of adjacent tissues [e.g. the endoderm in chick and mouse (Madabhushi and Lacy, 2011; Maretto et al., 2008; Varner and Taber, 2012), and the ectoderm in Drosophila (Haack et al., 2014)].
Myocardial cells engage in subduction during their medial migration
It was a surprise to find that during their medial migration, myocardial cells not only migrate by distinct modes at different stages, but also change their anatomic relationship with the endoderm (Figs 3-5). The revelation that myocardial precursors undergo such a process was possible because of high-resolution in toto imaging (Fig. 5; supplementary material Movie 5). Furthermore, our discovery that myocardial subduction was delayed and ineffective in the context of an S1pr2/Gα13 deficiency (Figs 3-5; supplementary material Fig. S3) suggests that endoderm convergence regulates the timing and efficiency of myocardial subduction. This idea is supported by the finding that myocardial subduction never occurred in the absence of endoderm (supplementary material Fig. S7). In addition, we found that not only cardiac mesodermal cells, but also other cells of the ALPM, undergo subduction (Fig. 9; supplementary material Fig. S7), suggesting that subduction is not specific to myocardial cells.
Notably, we also found that the myocardial population forms a two-layer structure during subduction (Fig. 5; supplementary material Movie 5). Future experiments involving new transgenic lines that label the nuclei and membranes of myocardial cells are expected to shed light on the changes in cell shape and the migratory behavior of myocardial cells at this stage. Although the mechanisms that promote myocardial subduction are unknown, we found that Fn fibrils displayed specific patterns of assembly around the myocardial cells, and the area between them and the endoderm and that S1pr2/Gα13-dependent endoderm convergence is crucial for this Fn assembly (Fig. 8). Thus, Fn assembly might be crucial only for establishing epithelial polarity, but perhaps also for facilitating myocardial subduction and migration. Indeed, we found that by the onset of myocardial subduction, the epithelial organization of the myocardial cells had already initiated (Fig. 7). This is much earlier than that was previously reported (Trinh and Stainier, 2004), suggesting that epithelial polarity is necessary for myocardial subduction. In addition, it is possible that Fn assembly facilitates mechanical contractility between the endoderm and myocardial precursors, a phenomenon that has been shown to promote tissue interactions (Lecuit et al., 2011; Miller and Davidson, 2013). In Gα13 morphants, Fn fibrils failed to assemble in the correct directions and were not enriched at the leading regions of myocardial populations (Fig. 8), and the epithelial polarity of myocardial cells was disrupted (Fig. 7; supplementary material Fig. S6). These outcomes could affect the subduction of myocardial cells and their subsequent medial migration.
Myocardial cells migrate independently of endoderm convergence during the final period of their medial migration
Notably, from 14 s, when the endoderm completes convergence, myocardial precursors continue to migrate medially towards the midline to form a single cell population, seemingly independent of convergent movement of the endoderm (Figs 3,4; supplementary material Movie 2). However, our experiments have not ruled out other potential roles of the endoderm in myocardial migration during this period. In particular, myocardial populations surrounded by Fn retain contact with endodermal sheet (Fig. 8). During this stage, the myocardial precursors are located between the endodermal sheet and the yolk syncytial layer (YSL) (Trinh and Stainier, 2004). Our data indicate that the endoderm stops migrating medially, and there is no evidence that the YSL moves towards the midline, although progenitor cell-mediated movement of internal yolk syncytial nuclei (iYSN) has been reported (Carvalho et al., 2009). Thus, there are no other cells/tissues adjacent to myocardial cells that migrate medially and could provide the pulling forces necessary to promote myocardial medial migration. Furthermore, our live imaging shows that myocardial cells display migratory protrusions at this stage (supplementary material Fig. S4C-F, Movie 3). Additionally, in addition to undergoing medial migration, myocardial cells were found to change their direction, angling towards endocardial precursors (Holtzman et al., 2007). This probably resulted from their intrinsic ability to migrate. Thus, the subduction and medial migration of myocardial cells after 14 s is probably due to their innate migration potential. Earlier studies in chick suggested that myocardial cells have little ability to migrate, and that endoderm movement drives their migration (Cui et al., 2009; Varner and Taber, 2012). However, a new study showed that chick myocardial cells instead exhibit active displacement and autonomous ventral movement, and that these changes are independent of the endoderm (Aleksandrova et al., 2015). It is thus possible that the ventral movement of these cells in chick is similar to the myocardial subduction we observed in zebrafish.
Of note, evidence also indicates that displacement of the extracellular matrix in the context of global tissue morphogenesis accounts for the movement of certain tissues (Bénazéraf et al., 2010; Cui et al., 2009; Zamir et al., 2008); this argues against cell-autonomous migration of the myocardial cells. Interestingly, our data show that the direction of Fn assembly aligns with that of myocardial-cell migration (Fig. 8), suggesting that these two events are linked. However, establishing whether Fn assembly resulted from or directs myocardial migration will require in vivo labeling and imaging of Fn. This is not feasible with the technologies currently available for zebrafish.
In addition, we found that, although myocardial cells in S1pr2/Gα13-deficient embryos eventually migrated to the ventral side of the endoderm, they inevitably failed to migrate towards the midline (Figs 3,4; supplementary material Fig. S3). This could be due to defects in epithelial polarity of myocardial cells in context of S1pr2/Gα13-deficiency (Fig. 7). However, we cannot exclude the involvement of other factors, such as endocardial cells. Similar to myocardial cells, two populations of endocardial precursors migrate towards, and merge at, the midline, and then migrate towards the myocardial cells to form the heart cone (Bussmann et al., 2007). It would be interesting to assess whether the medial migration of endocardial cells depends on endoderm convergence, whether endocardial cells also undergo subduction and what the consequences for myocardial migration are when endocardial migration is defective in S1pr2/Gα13-deficient embryos.
In summary, our work shows that during heart-tube formation, myocardial cells exhibit three distinct migratory modes, and their migration depends on endoderm convergence during early stage, but not during the late stage. Similarly, a recent study in Drosophila showed that cardiac cells initially depend on the adjacent ectoderm to migrate, but exhibit autonomous and active migration at later stages (Haack et al., 2014). Our results have significant implications for the interplay of tissues in organ development. In particular, many congenital heart defects, such as Cornelia De Lange and DiGeorge syndromes (Arnold et al., 2006; Muto et al., 2011), also display endoderm defects. Understanding the interactions between the endoderm and the cardiac mesoderm might shed light on the causes of such disease.
MATERIALS AND METHODS
Zebrafish strains and maintenance
AB*/Tuebingen, transgenic Tg(myl7:EGFP) (Huang et al., 2003), Tg(sox17:EGFP) (Mizoguchi et al., 2008), Tg(hand2:GFP)pd24 (Yin et al., 2010), Tg(nkx2.5:ZsYellow) (Zhou et al., 2011), Tg(nkx2.5:Kaede) (Guner-Ataman et al., 2013) and milm93 zebrafish strains (Kupperman et al., 2000) were used. Embryos were obtained by natural mating, maintained at 28.5°C and, unless specified otherwise, were staged according to morphology or hours post fertilization (hpf), as described previously (Kimmel et al., 1995).
Generation of transgenic lines
The transgenic lines Tg(sox17:memCherry), Tg(sox17:mCherry-2A-gna13a), Tg(hsp70:gna13a), Tg(myl7:memGFP) and Tg(nkx2.5:lifeactGFP) were generated using a Tol2-based Multi-Site Gateway system (Life Technologies) (Kwan et al., 2007; Villefranc et al., 2007). All transgenic constructs were assembled using entry clones kindly provided by Chi-Bin Chien and Kristen Kwan (University of Utah, USA), unless specified otherwise. A 5′-entry clone containing a sox17 mini-promoter (Woo et al., 2012) was used to express genes specifically in the endoderm. A 5′-entry clone containing an nkx2.5 promoter (Choe et al., 2013) was used to express genes specifically in myocardial cells. A middle clone encoding mCherry, and a 3′-entry clone encoding the 2A peptide, a zebrafish gna13a-coding region (morpholino-insensitive), and SV40, was created to express Gα13a (mCherry is used to identify lines expressing Gα13a). Middle-entry clones encoding the zebrafish gna13a and lifeact-GFP coding region were created using the pENTR Directional TOPO Cloning Kit (Life Technologies). Tg(hsp70:gna13a) line was generated using a destination vector that contains a myl7:GFP cassette (GFP expression in the heart was used to identify embryos that contain the gna13a transgene). Transgenic lines were established using standard techniques as described previously (Xu et al., 2014). The founders were bred to generate multiple stable lines.
Whole-mount in situ hybridization (WISH) and immunofluorescence (IF)
Standard methods were used for WISH and IF. Please see supplementary materials methods for further details.
Microscopy, time-lapse imaging and image analysis
Time-lapse imaging was performed as described previously (Ye and Lin, 2013). Please see supplementary materials methods for a detailed description of microscopy, imaging and image analysis.
Morpholino (MO) injection
The previously validated MOs targeting the following genes were injected into embryos at 1-cell stage: gna13a and gna13b (2-3 ng each) (Lin et al., 2005), s1pr2/mil (15 ng) (Kawahara et al., 2009) and sox32 (4 ng) (Wong et al., 2012).
Statistical analysis
Data were compiled from two to three independent experiments and are presented as mean±s.e.m. Statistical analyses were performed using unpaired Student's two-tailed t-test and unequal variance, and P<0.05 was considered significant.
Supplementary Material
Acknowledgements
We are grateful to Caroline Burns and Geoffrey Burns (Harvard Medical School) for providing the Tg(nkx2.5:ZsYellow) and Tg(nkx2.5:Kaede) lines; Sean Megason and Fengzhu Xiong (Harvard Medical School) for the mounting mould; Stephanie Woo (University of California, San Francisco) for the sox17 promoter 5′ entry clone; Gage Crump (University of Southern California) for the nkx2.5 promoter 5′ entry clone; Carl-Philipp Heisenberg for lifeact-GFP entry clone (IST Austria); and Hui Xu and Baosheng Xie (University of Iowa) for generating the Tg(hsp70:gna13a) line and the Tg(nkx2.5:lifeact-GFP) construct, respectively.
Footnotes
Author contributions
F.L. and D.Y. conceived the ideas and designed experiments; D.Y., H.X. and B.H. performed the experiments; F.L. wrote the manuscript.
Funding
This work was supported by grants from the American Heart Association [12GRNT11670009, 2012]; March of Dimes Foundation Research [2013]; and the National Science Foundation [IOS-1354457] (all to F.L.).
Contributor Information
Ding Ye, Department of Anatomy and Cell Biology, Carver College of Medicine, University of Iowa, 1-400 Bowen Science Building, 51 Newton Road, Iowa City, IA 52242-1109, USA.
Huaping Xie, Department of Anatomy and Cell Biology, Carver College of Medicine, University of Iowa, 1-400 Bowen Science Building, 51 Newton Road, Iowa City, IA 52242-1109, USA.
Bo Hu, Department of Anatomy and Cell Biology, Carver College of Medicine, University of Iowa, 1-400 Bowen Science Building, 51 Newton Road, Iowa City, IA 52242-1109, USA.
Fang Lin, Department of Anatomy and Cell Biology, Carver College of Medicine, University of Iowa, 1-400 Bowen Science Building, 51 Newton Road, Iowa City, IA 52242-1109, USA.
References
- Aleksandrova, A., Czirok, A., Kosa, E., Galkin, O., Cheuvront, T. J. and Rongish, B. J. (2015). The endoderm and myocardium join forces to drive early heart tube assembly. Dev. Biol. 404, 40-54. 10.1016/j.ydbio.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrée, B., Duprez, D., Vorbusch, B., Arnold, H.-H. and Brand, T. (1998). BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech. Dev. 70, 119-131. 10.1016/S0925-4773(97)00186-X [DOI] [PubMed] [Google Scholar]
- Arnold, J. S., Werling, U., Braunstein, E. M., Liao, J., Nowotschin, S., Edelmann, W., Hebert, J. M. and Morrow, B. E. (2006). Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development 133, 977-987. 10.1242/dev.02264 [DOI] [PubMed] [Google Scholar]
- Arrington, C. B. and Yost, H. J. (2009). Extra-embryonic syndecan 2 regulates organ primordia migration and fibrillogenesis throughout the zebrafish embryo. Development 136, 3143-3152. 10.1242/dev.031492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkers, J. (2011). Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 91, 279-288. 10.1093/cvr/cvr098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénazéraf, B., Francois, P., Baker, R. E., Denans, N., Little, C. D. and Pourquié, O. (2010). A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466, 248-252. 10.1038/nature09151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann, J., Bakkers, J. and Schulte-Merker, S. (2007). Early endocardial morphogenesis requires Scl/Tal1. PLoS Genet. 3, e140. 10.1371/journal.pgen.0030140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, L., Stuhmer, J., Bois, J. S., Kalaidzidis, Y., Lecaudey, V. and Heisenberg, C.-P. (2009). Control of convergent yolk syncytial layer nuclear movement in zebrafish. Development 136, 1305-1315. 10.1242/dev.026922 [DOI] [PubMed] [Google Scholar]
- Choe, C. P., Collazo, A., Trinh le, A., Pan, L., Moens, C. B. and Crump, J. G. (2013). Wnt-dependent epithelial transitions drive pharyngeal pouch formation. Dev. Cell 24, 296-309. 10.1016/j.devcel.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, C., Cheuvront, T. J., Lansford, R. D., Moreno-Rodriguez, R. A., Schultheiss, T. M. and Rongish, B. J. (2009). Dynamic positional fate map of the primary heart-forming region. Dev. Biol. 332, 212-222. 10.1016/j.ydbio.2009.05.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, N. B. and Rosa, F. M. (2001). Cell autonomous commitment to an endodermal fate and behaviour by activation of Nodal signalling. Development 128, 3937-3947. [DOI] [PubMed] [Google Scholar]
- Evans, S. M., Yelon, D., Conlon, F. L. and Kirby, M. L. (2010). Myocardial lineage development. Circ. Res. 107, 1428-1444. 10.1161/CIRCRESAHA.110.227405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito-Aguilar, Z. V., Riley, H. E. and Yelon, D. (2010). Hand2 ensures an appropriate environment for cardiac fusion by limiting Fibronectin function. Development 137, 3215-3220. 10.1242/dev.052225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guner-Ataman, B., Paffett-Lugassy, N., Adams, M. S., Nevis, K. R., Jahangiri, L., Obregon, P., Kikuchi, K., Poss, K. D., Burns, C. E. and Burns, C. G. (2013). Zebrafish second heart field development relies on progenitor specification in anterior lateral plate mesoderm and nkx2.5 function. Development 140, 1353-1363. 10.1242/dev.088351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack, T., Schneider, M., Schwendele, B. and Renault, A. D. (2014). Drosophila heart cell movement to the midline occurs through both cell autonomous migration and dorsal closure. Dev. Biol. 396, 169-182. 10.1016/j.ydbio.2014.08.033 [DOI] [PubMed] [Google Scholar]
- Holtzman, N. G., Schoenebeck, J. J., Tsai, H.-J. and Yelon, D. (2007). Endocardium is necessary for cardiomyocyte movement during heart tube assembly. Development 134, 2379-2386. 10.1242/dev.02857 [DOI] [PubMed] [Google Scholar]
- Huang, C. J., Tu, C. T., Hsiao, C. D., Hsieh, F. J. and Tsai, H. J. (2003). Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 228, 30-40. 10.1002/dvdy.10356 [DOI] [PubMed] [Google Scholar]
- Kawahara, A., Nishi, T., Hisano, Y., Fukui, H., Yamaguchi, A. and Mochizuki, N. (2009). The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524-527. 10.1126/science.1167449 [DOI] [PubMed] [Google Scholar]
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. and Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Kupperman, E., An, S., Osborne, N., Waldron, S. and Stainier, D. Y. R. (2000). A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 406, 192-195. 10.1038/35018092 [DOI] [PubMed] [Google Scholar]
- Kwan, K. M., Fujimoto, E., Grabher, C., Mangum, B. D., Hardy, M. E., Campbell, D. S., Parant, J. M., Yost, H. J., Kanki, J. P. and Chien, C.-B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088-3099. 10.1002/dvdy.21343 [DOI] [PubMed] [Google Scholar]
- Lecuit, T., Lenne, P.-F. and Munro, E. (2011). Force generation, transmission, and integration during cell and tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 27, 157-184. 10.1146/annurev-cellbio-100109-104027 [DOI] [PubMed] [Google Scholar]
- Lin, F., Sepich, D. S., Chen, S., Topczewski, J., Yin, C., Solnica-Krezel, L. and Hamm, H. (2005). Essential roles of Ga12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J. Cell. Biol. 169, 777-787. 10.1083/jcb.200501104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough, J. and Sugi, Y. (2000). Endoderm and heart development. Dev. Dyn. 217, 327-342. [DOI] [PubMed] [Google Scholar]
- Madabhushi, M. and Lacy, E. (2011). Anterior visceral endoderm directs ventral morphogenesis and placement of head and heart via BMP2 expression. Dev. Cell 21, 907-919. 10.1016/j.devcel.2011.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto, S., Müller, P.-S., Aricescu, A. R., Cho, K. W. Y., Bikoff, E. K. and Robertson, E. J. (2008). Ventral closure, headfold fusion and definitive endoderm migration defects in mouse embryos lacking the fibronectin leucine-rich transmembrane protein FLRT3. Dev. Biol. 318, 184-193. 10.1016/j.ydbio.2008.03.021 [DOI] [PubMed] [Google Scholar]
- Matsui, T., Raya, Á., Callol-Massot, C., Kawakami, Y., Oishi, I., Rodriguez-Esteban, C. and Belmonte, J. C. I. (2007). miles-apart-Mediated regulation of cell–fibronectin interaction and myocardial migration in zebrafish. Nat. Clin. Pract. Cardiovasc. Med. 4 Suppl. 1, S77-S82. 10.1038/ncpcardio0764 [DOI] [PubMed] [Google Scholar]
- Miller, C. J. and Davidson, L. A. (2013). The interplay between cell signalling and mechanics in developmental processes. Nat. Rev. Genet. 14, 733-744. 10.1038/nrg3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi, T., Verkade, H., Heath, J. K., Kuroiwa, A. and Kikuchi, Y. (2008). Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development 135, 2521-2529. 10.1242/dev.020107 [DOI] [PubMed] [Google Scholar]
- Muto, A., Calof, A. L., Lander, A. D. and Schilling, T. F. (2011). Multifactorial origins of heart and gut defects in nipbl-deficient zebrafish, a model of Cornelia de Lange Syndrome. PLoS Biol. 9, e1001181. 10.1371/journal.pbio.1001181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita, N., Bielinska, M. and Wilson, D. B. (1997). Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev. Biol. 189, 270-274. 10.1006/dbio.1997.8684 [DOI] [PubMed] [Google Scholar]
- Nascone, N. and Mercola, M. (1995). An inductive role for the endoderm in Xenopus cardiogenesis. Development 121, 515-523. [DOI] [PubMed] [Google Scholar]
- Osborne, N., Brand-Arzamendi, K., Ober, E. A., Jin, S.-W., Verkade, H., Holtzman, N. G., Yelon, D. and Stainier, D. Y. R. (2008). The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr. Biol. 18, 1882-1888. 10.1016/j.cub.2008.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter, J. F., Alexander, J., Rodaway, A., Yelon, D., Patient, R., Holder, N. and Stainier, D. Y. R. (1999). Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 13, 2983-2995. 10.1101/gad.13.22.2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist, G. C. (1970). Cardia bifida in chick embryos: anterior and posterior defects produced by transplanting tritiated thymidine-labeled grafts medial to the heart-forming regions. Teratology 3, 135-142. 10.1002/tera.1420030205 [DOI] [PubMed] [Google Scholar]
- Sakaguchi, T., Kikuchi, Y., Kuroiwa, A., Takeda, H. and Stainier, D. Y. R. (2006). The yolk syncytial layer regulates myocardial migration by influencing extracellular matrix assembly in zebrafish. Development 133, 4063-4072. 10.1242/dev.02581 [DOI] [PubMed] [Google Scholar]
- Schultheiss, T. M., Xydas, S. and Lassar, A. B. (1995). Induction of avian cardiac myogenesis by anterior endoderm. Development 121, 4203-4214. [DOI] [PubMed] [Google Scholar]
- Shoji, W. and Sato-Maeda, M. (2008). Application of heat shock promoter in transgenic zebrafish. Dev. Growth Differ. 50, 401-406. 10.1111/j.1440-169X.2008.01038.x [DOI] [PubMed] [Google Scholar]
- Shook, D. R., Majer, C. and Keller, R. (2002). Urodeles remove mesoderm from the superficial layer by subduction through a bilateral primitive streak. Dev. Biol. 248, 220-239. 10.1006/dbio.2002.0718 [DOI] [PubMed] [Google Scholar]
- Shook, D. R., Majer, C. and Keller, R. (2004). Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev. Biol. 270, 163-185. 10.1016/j.ydbio.2004.02.021 [DOI] [PubMed] [Google Scholar]
- Staudt, D. and Stainier, D. (2012). Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu. Rev. Genet. 46, 397-418. 10.1146/annurev-genet-110711-155646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh, L. A. and Stainier, D. Y. R. (2004). Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell 6, 371-382. 10.1016/S1534-5807(04)00063-2 [DOI] [PubMed] [Google Scholar]
- Trinh, L. A., Yelon, D. and Stainier, D. Y. R. (2005). Hand2 regulates epithelial formation during myocardial differentiation. Curr. Biol. 15, 441-446. 10.1016/j.cub.2004.12.083 [DOI] [PubMed] [Google Scholar]
- Varner, V. D. and Taber, L. A. (2012). Not just inductive: a crucial mechanical role for the endoderm during heart tube assembly. Development 139, 1680-1690. 10.1242/dev.073486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villefranc, J. A., Amigo, J. and Lawson, N. D. (2007). Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077-3087. 10.1002/dvdy.21354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, K. S., Rehn, K., Palencia-Desai, S., Kohli, V., Hunter, W., Uhl, J. D., Rost, M. S. and Sumanas, S. (2012). Hedgehog signaling is required for differentiation of endocardial progenitors in zebrafish. Dev. Biol. 361, 377-391. 10.1016/j.ydbio.2011.11.004 [DOI] [PubMed] [Google Scholar]
- Woo, S., Housley, M. P., Weiner, O. D. and Stainier, D. Y. R. (2012). Nodal signaling regulates endodermal cell motility and actin dynamics via Rac1 and Prex1. J. Cell Biol. 198, 941-952. 10.1083/jcb.201203012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H., Ye, D., Behra, M., Burgess, S., Chen, S. and Lin, F. (2014). Gb1 controls collective cell migration by regulating the protrusive activity of leader cells in the posterior lateral line primordium. Dev. Biol. 385, 316-327. 10.1016/j.ydbio.2013.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, D. and Lin, F. (2013). S1pr2/Gα13 signaling controls myocardial migration by regulating endoderm convergence. Development 140, 789-799. 10.1242/dev.085340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelon, D., Horne, S. A. and Stainier, D. Y. R. (1999). Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 214, 23-37. 10.1006/dbio.1999.9406 [DOI] [PubMed] [Google Scholar]
- Yelon, D., Ticho, B., Halpern, M. E., Ruvinsky, I., Ho, R. K., Silver, L. M. and Stainier, D. Y. (2000). The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development 127, 2573-2582. [DOI] [PubMed] [Google Scholar]
- Yin, C., Kikuchi, K., Hochgreb, T., Poss, K. D. and Stainier, D. Y. (2010). Hand2 regulates extracellular matrix remodeling essential for gut-looping morphogenesis in zebrafish. Dev. Cell 18, 973-984. 10.1016/j.devcel.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir, E. A., Rongish, B. J., Little, C. D. and Hogan, B. (2008). The ECM moves during primitive streak formation—computation of ECM versus cellular motion. PLoS Biol. 6, e247. 10.1371/journal.pbio.0060247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., Cashman, T. J., Nevis, K. R., Obregon, P., Carney, S. A., Liu, Y., Gu, A., Mosimann, C., Sondalle, S., Peterson, R. E., et al. (2011). Latent TGF-β binding protein 3 identifies a second heart field in zebrafish. Nature 474, 645-648. 10.1038/nature10094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.