Figure 3.
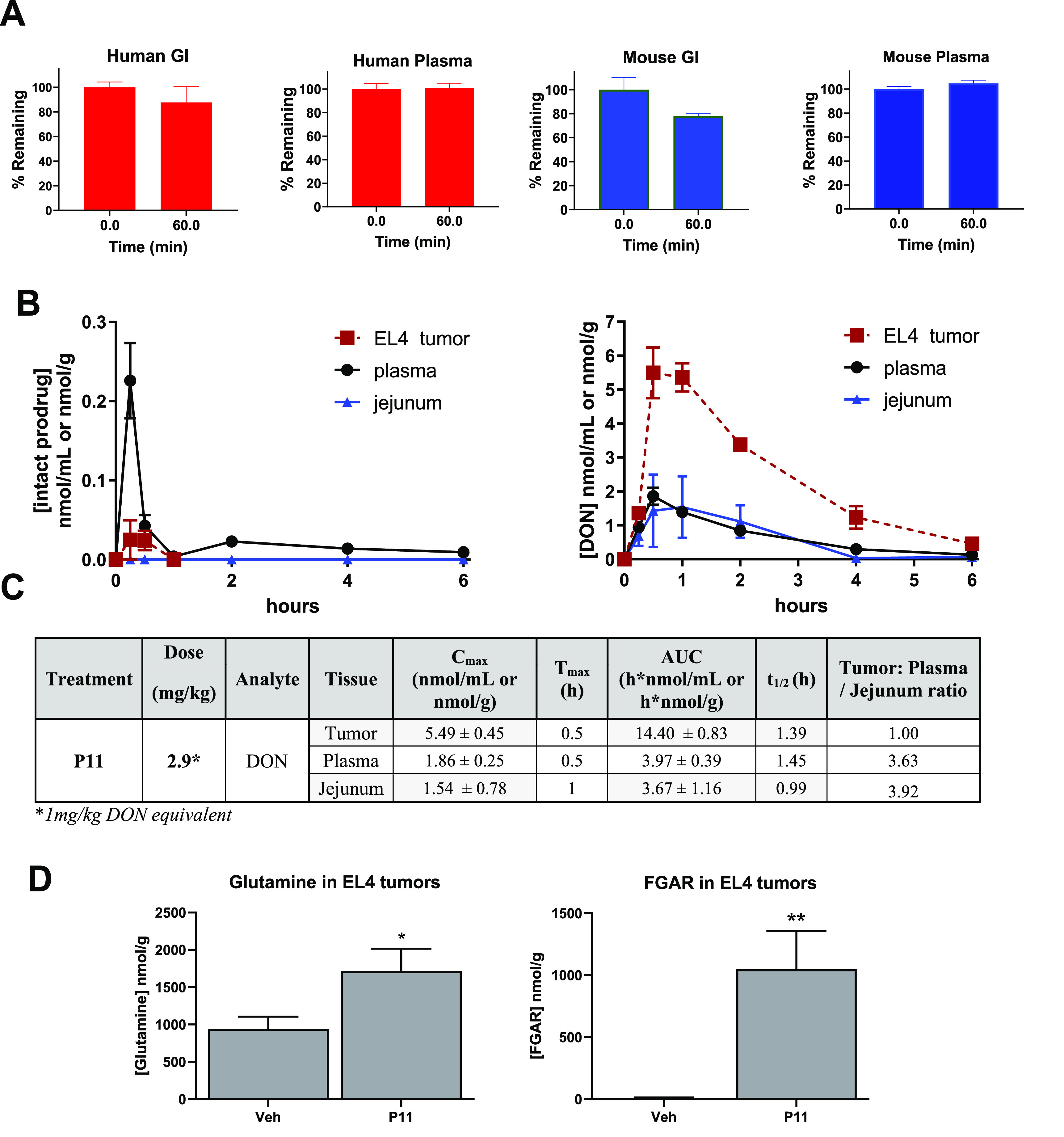
Stability, pharmacokinetic analysis, and tumor target engagement of P11. (A) Stability of P11 in human GI microsomes and plasma and mouse GI homogenate and mouse plasma. (B, C) P11 (2.9 mg/kg) was administered subcutaneously (SC) to C57BL/6/CES1–/– mice bearing EL4 tumors, and tissues were harvested and analyzed for (B) intact (P11) and released DON in tumor, plasma, and jejunum. (C) PK parameters of released DON. Data expressed as mean ± SEM, n = 3. EL4 tumors collected from these mice at Tmax were used for quantification of (D) tumor glutamine and FGAR quantification at 30 min post dose for target engagement evaluation (mean ± SD; ∗, p > 0.01; ∗∗, p > 0.001; unpaired two-tailed t test).
