Scheme 1. Synthesis of Prodrugs P1–P6.
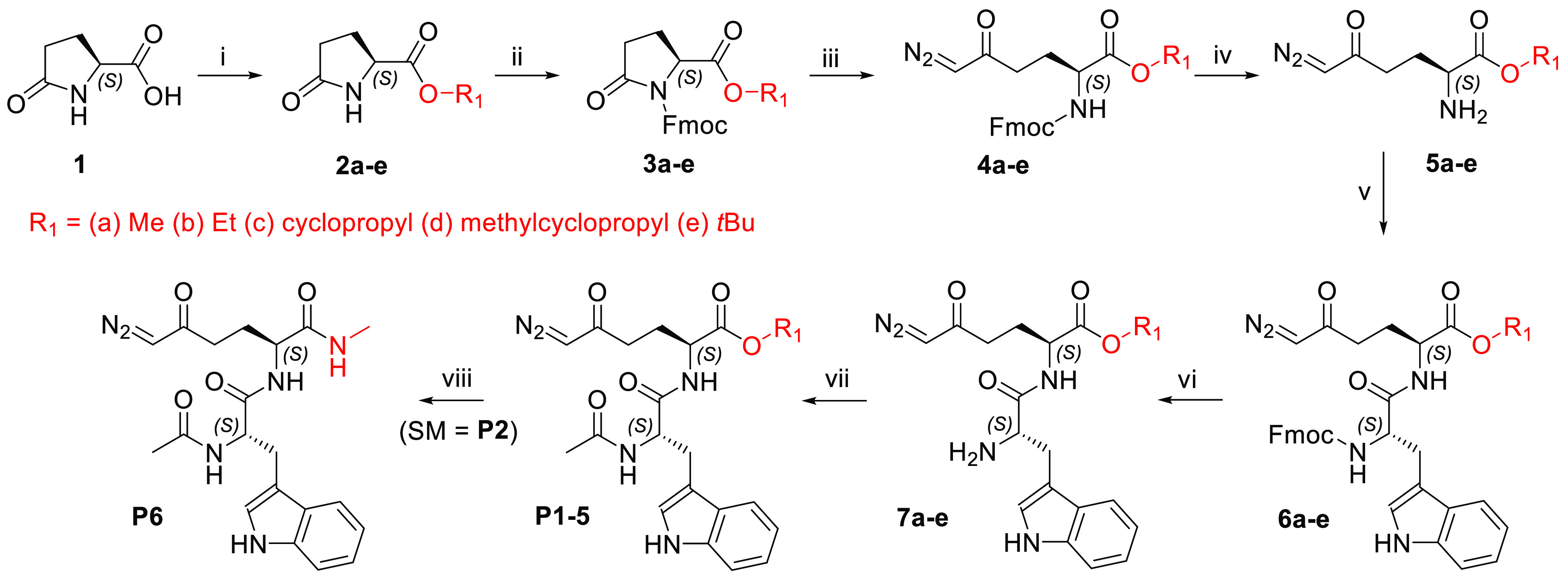
Reagents and conditions: (i) for 2a and 2b, R1–OH (MeOH or EtOH), SOCl2, 0 °C to rt, 16 h, 93–98%; for 2c and 2d, R1–OH (cyclopropanol or cyclopropylmethanol), DCC, DMAP, DCM, rt, 16 h, 96–97%; for 2e, tert-butyl acetate, perchloric acid, rt, 48 h, 90%; (ii) Fmoc-Cl, LiHMDS, THF, −78 °C to rt, 16 h, 63–95%; (iii) TMSCHN2, n-BuLi, THF, −78 °C, 3 h, 47–67%; (iv) piperidine, DCM, rt, 3 h, 49–67%; (v) Fmoc-l-Trp-OH, HATU, DIPEA, DCM, or DCM/DMF 4:1, 0 °C to rt, 1.5 h, 73–95%; (vi) diethylamine, DCM, rt, 3–6 h, 90–95%; (vii) Ac2O, py, DMF, rt, 3–15 h, 66–92%; (viii) (SM = P2), 2 M methylamine in MeOH, 60 °C, 20 h, 65%.
