Scheme 3. Synthesis of Prodrugs P10–P21.
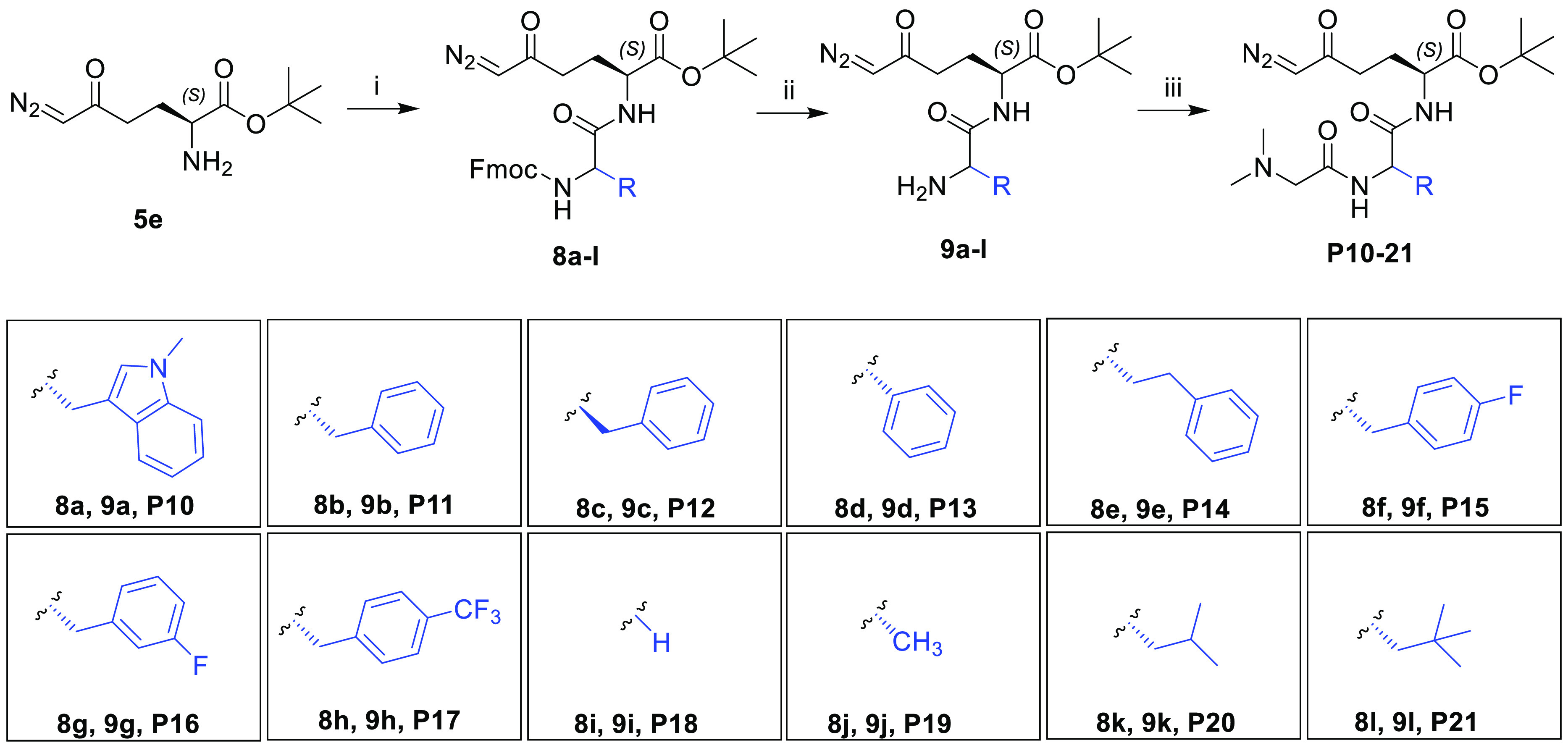
Reagents and conditions: (i) Fmoc-AA-OH (8a, Fmoc-l-Trp(N-Me)-OH; 8b, Fmoc-l-Phe-OH; 8c, Fmoc-d-Phe-OH; 8d, Fmoc-l-Phg-OH; 8e, Fmoc-l-HomoPhe-OH; 8f, Fmoc-l-Phe(4-F)-OH; 8g, Fmoc-l-Phe(3-F)-OH; 8h, Fmoc-l-Phe(4-CF3)-OH; 8i, Fmoc-Gly-OH; 8j, Fmoc-l-Ala-OH·H2O; 8k, Fmoc-l-Leu-OH; 8l, Fmoc-l-Ala(β-tBu)-OH), HATU, DIPEA, DCM, 0 °C to rt, 1.5–16 h, 68–98%; (ii) diethylamine, DCM, rt, 1.5–7 h, 76–96%; (iii) for P10, P11, P18, and P19, dimethylglycine, HATU, DIPEA, DCM, or DMF, 0 °C to rt, 1.5–2.5 h, 64–73%; for P12–P17, P20, and P21, 2,5-dioxopyrrolidin-1-yl dimethylglycinate, DCM, rt, 2–20 h, 51–92%.
