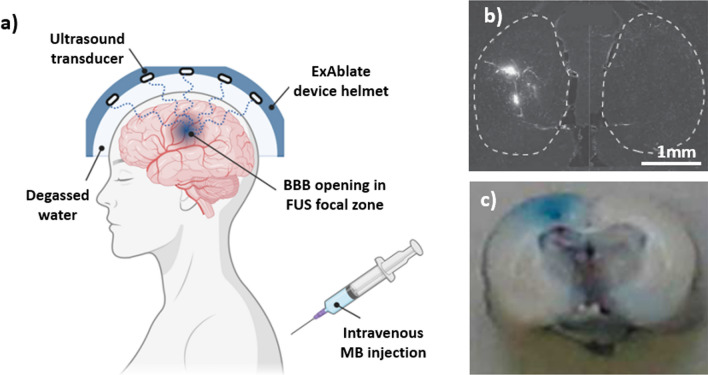
Fig. 5.
Schematic representation of blood–brain barrier opening in Alzheimer’s disease patient in vivo and patient-derived model in vitro. a Schematic of a magnetic resonance (MR)-guided ExAblate device used in the first successful blood–brain barrier (BBB) opening in Alzheimer’s disease (AD) patients. System consists of a hemispherical helmet lined with > 1000 independent transducer elements delivering low frequency ultrasound treatment to the prescribed target. The helmet is positioned in the specialised MRI bed with stereotaxic frame and the space between patient’s head and the helmet filled with degassed water for acoustic coupling. Microbubble (MB) administration is carried out using repeated bolus injection or a continuous infusion. Reversible BBB opening occurs in the defined ultrasound focal zone. Figure taken from [161]. b fluorescence images of FUS treated at US pressure of 0.84 MPa mouse brains (left side) compared to non-treated (right side), each injected with 500 kDa size dextran’s. Dashed lines indicated boundaries of the hippocampal regions. Figure taken from Chen et al. [142]. c Detection of BBB opening in rat brain, with left hemisphere sonicated at an intracranial pressure amplitude of 1.25 MPa in the presence of microbubbles at a dosage of 0.1 mL/kg and no treatment to the right side. Macroscopic inspection of 2–3 mm thick coronal section (front side of the brain) showed penetration of Evans Blue (molecular weight of 960.8 Da) into the underlying cortex. Figure taken from Alonso et al. [143]