Abstract
Background
Despite numerous national depression care guidelines (DCGs), suboptimal antidepressant treatment may occur. We examined DCG concordance and depression treatment outcomes in psychiatric settings.
Methods
We evaluated treatment received and outcomes of 128 psychiatric out- and inpatients participating in the PEGAD (Pharmacoepidemiology and Pharmacogenetics of Antidepressant Treatment for Depressive Disorders) study at baseline, two weeks, and eight weeks using interviews and questionnaires. Inclusion criteria were ICD-10 diagnosis of a depressive disorder, a Patient Health Questionnaire-9 symptom (PHQ-9) score ≥ 10, and a new antidepressant prescribed. The primary outcome of the study was within-individual change in PHQ-9 scores.
Results
At baseline, patients had predominately recurrent (83%) and in 19% treatment-resistant depression (TRD). The median preceding duration of the current episode was 6.5 months. At eight weeks, 85% of the patients (n = 107) used a DCG-concordant antidepressant dose. However, due to the scarcity of antidepressant combinations and augmentations, fewer TRD than non-TRD patients (25% vs. 84%, p < 0.005) received adequate antidepressant treatment. Additionally, one-third of the patients received inadequate follow-up. Overall, only 53% received treatment compatible with DCG recommendations for adequate pharmacotherapy and follow-up. The mean decline in PHQ-9 scores (-3.8 ± SD 5.7) was significant (p < 0.0005). Nearly 40% of the patients reached a subthreshold level of depression (PHQ-9 < 10), predicted by a lower baseline PHQ-9 score, recurrent depression, and female sex. However, 45% experienced no significant clinical improvement (PHQ-9 score reduction < 20%).
Conclusions
Our findings suggest that inadequate treatment continues to occur in psychiatric care settings, particularly for TRD patients.
Keywords: Depression, Psychiatric care, Antidepressant, Treatment adequacy, Treatment outcome
Introduction
Depression treatment should strive for full remission. The essential treatment option of antidepressants (ADs) is endorsed by numerous national depression care guidelines (DCGs), e.g. [1–4], particularly in moderate to severe depression, as robust evidence indicates their effectiveness and safety [5]. AD monotherapy is an evidence-based treatment option for acute depression and relapse prevention, which, following Finnish DCG recommendations [6], is predominately carried out in primary care. Patients with psychotic or treatment-resistant depression (TRD, defined as depression not responding to two or more adequately conducted AD monotherapy trials; [7]) and significant psychiatric comorbidity, functional disability, or suicidality should be referred to psychiatric care as they often require more complex pharmacotherapy, including AD combinations and augmentation with lithium or an antipsychotic [8–14]. To facilitate therapeutic decisions based on outcome predictors and observed treatment responses [15–17], DCGs endorse measurement-based care (MBC), i.e., routine monitoring of clinician- or patient-rated depression measures [18–20]. Thus, MBC provides clinicians with evidence-based tools for individualized depression treatment, additionally associated with better outcomes [19–21].
However, DCG-discordant or individually sub-optimized depression treatment is still an acknowledged problem in primary and psychiatric care settings [22–28]. Vigo et al. [29] concluded that only 10% of patients with major depressive disorder (MDD) received an adequate combination and implementation of pharmacotherapy and psychotherapy. Specifically, the main shortcomings of pharmacotherapy were underutilization and inadequate clinical monitoring of responses and side effects. A nationally representative survey study from the USA [30] found that less than one-third of non-remitted, AD-treated patients received augmentation treatment. One naturalistic European study [31] evaluating DCG adherence in outpatient care noted a scarcity of AD dose and medication changes, regardless of treatment outcome. Moreover, primary and specialized psychiatric care patients have been described as unexpectedly similar in depressive symptoms and depression severity [27, 32, 33], indicating possible clinical practice conflicting with DCG recommendations concerning referral to specialized care. Previously reported rates of depression treatment adequacy in psychiatric care have shown a wide variation between 31.0% and 94.4% [23, 24, 34–36]. However, there are very few studies investigating the concordance of treatments provided in psychiatric settings with DCG recommendations during the current era of guidelines and widespread AD use.
Earlier Finnish studies have also shown quality-of-care problems in depression treatment, e.g., treatment initiation delays, suboptimal treatment intensity, continuity challenges, and an indistinct division of labour between primary and psychiatric care [37–40]. Lähteenvuo et al. [41] recently reported on a nationwide register-based cohort that AD monotherapy was the most frequently initiated treatment, even among those TRD patients progressing to a fifth treatment trial after four previous monotherapies. This finding indicates likely non-adherence to DCG recommendations, as pharmacological combination or augmentation strategies are recommended after two failed monotherapy trials. Therefore, a more detailed study in a smaller sample is vital to broadening the understanding of current depression treatment practices and outcomes in psychiatric care settings. The Finnish DCG, first published in 2004, was recently updated in 2020. However, most studies published on the clinical practice of depression treatment in the Finnish public health care system date back more than a decade. Little is known about how treatment practices may have changed over the years. Additionally, when considering factors impacting depression care practice, we cannot ignore the 40% reduction in Finnish psychiatric hospital beds in recent years [42] and the increasing shortage of psychiatrists and other healthcare professionals in the public sector.
In this observational eight-week follow-up study, we aimed to examine Finnish DCG adherence in the psychiatric care settings of Helsinki University Hospital, Finland’s largest hospital district. Specifically, we aimed to describe (1) the clinical characteristics, prior course of illness, and treatment history of patients currently referred to psychiatric care, (2) the treatment received, focusing on AD use, and (3) the short-term treatment outcome. We expected treatment practice to align with the current recommendations, including appropriate AD dosage, treatment duration, and follow-up.
Methods
Study design and setting
This eight-week observational prospective cohort study is part of the PEGAD (Pharmacoepidemiology and pharmacogenetics of antidepressant treatment for depressive disorders) project, conducted within Helsinki University Hospital´s divisions of Acute Psychiatry, Mood Disorders, and Geropsychiatry. The Helsinki University Hospital catchment area provides adult psychiatric out- and inpatient services to Espoo, Kauniainen, Kirkkonummi, Vantaa, and Kerava, and geropsychiatric services to Helsinki and neighbouring cities, facilities from which clinicians recruited study patients between August 2018 and November 2019. The Ethics Committee of Helsinki University Hospital and the Finnish Medicines Agency (FIMEA) approved the study protocol. All recruited patients gave written informed consent. The study was based on clinical diagnoses by attending psychiatrists responsible for providing patients’ usual care. During follow-up patients received treatment as usual for their depressive disorder.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) an ICD-10 (International Statistical Classification of Diseases and Related Health Problems 10th Revision) [43] diagnosis of Depressive episode (F32) or Recurrent depressive disorder (F33), (2) a Patient Health Questionnaire-9 (PHQ-9) [44] score ≥ 10, (3) a new AD prescribed, and (4) age ≥ 18 years. The exclusion criteria were (1) a principal clinical diagnosis other than depression, (2) current psychotic symptoms, (3) immediate suicide risk, and (4) involuntary hospitalization.
Evaluation and scales
We evaluated patients at three time points: baseline, two weeks, and eight weeks.
At baseline, research nurses collected sociodemographic and clinical data using interviews and instructed patients to answer the self-report questionnaires. Symptom and treatment history of current major depressive episode (MDE) was based on patients’ recollection. Patients were asked structured questions to estimate the time points for initial signs and symptoms reaching the level of clinical depression. For each AD reportedly used before study participation, we enquired when the patients had used it, the duration of its use, its highest dosage, and the reason for its discontinuation.
The Columbia–Suicide Severity Rating Scale (C-SSRS) [45] was used to identify and rate suicidal ideation and behaviour occurring after the initial screening and admittance to the study.
The self-report scales included the PHQ-9, the Overall Anxiety and Impairment Scale (OASIS) [46], the Mood Disorder Scale (MDQ) [47], the Snaith-Hamilton Pleasure scale (SHAPS) [48], the Sheehan Disability Scale (SDS) [49], the Alcohol Use Disorders Identification Test (AUDIT) [50], questions on illegal drug use, the Fagerström Test for Nicotine Dependence (FTND) [51], and the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD) [52].
At two weeks, patients received an email inquiry asking if they still used the AD described at baseline. Additionally, patients were asked to fill in the PHQ-9, OASIS, and MDQ.
At eight weeks, research nurses conducted interviews concerning treatment received and patient adherence during follow-up. Drug use was specified by asking about all current psychiatric and somatic medication (dosage and time of last use) and psychiatric medication used during the preceding week. The patients also filled in the PHQ-9, OASIS, SHAPS, SDS, and MDQ.
Primary outcome
The primary outcome of depression treatment was the within-individual change in PHQ-9 symptom scores between baseline and the eight-week time point. We also divided patients into groups according to baseline depression severity, examining the change in PHQ-9 symptom scores between baseline and the eight-week time point specifically in each group.
Minimally adequate treatment
The overall treatment received during the study was classified as “minimally adequate” for non-TRD patients when the following criteria were met: (1) receiving AD for two months and having an adequate treatment dose (defined by the Finnish DCG) at the eight-week time point and (2) including at least two follow-up visits at the treating facility. “Minimally adequate” treatment for TRD patients had the additional criterion of receiving AD combination or pharmacological augmentation treatment.
Statistical analysis
As the amount of missing symptom scale data was small (data available on request), we used the mean substitution method to address missing data. We used the Chi-square and Fisher Exact tests to examine associations between categorical variables and the Mann-Whitney test to compare continuous variables between the ICD-10 specific diagnosis groups. The effects of independent baseline variables on the likelihood of reaching a PHQ-9 value < 10 at the eight-week time point were examined using a multivariate logistic regression model. We performed all analyses using the SPSS program [53].
Results
Patient sampling
We excluded three of the 131 patients recruited for technical reasons, resulting in a baseline patient number of 128. Ninety-one patients (71.1%) provided data at two weeks and 107 (83.6%) at eight weeks. Most (n = 113; 88.3%) were outpatients at the time of recruitment, and the rest (n = 15; 11.7%) were inpatients. All follow-ups were conducted in outpatient care. A minority of participants (n = 16; 12.5%) were psychogeriatric patients (age > 65 years).
Patient characteristics
The majority (n = 85; 66.4%) of the 128 patients were women, predominantly (n = 54; 63.5%) unmarried, separated, or widowed. Women had a higher educational level (p = 0.047) and were more likely to be a part of the active workforce (p < 0.001) than men (Table 1). Patients who dropped out after the baseline evaluation (n = 21) did not differ in their baseline clinical characteristics from those remaining in follow-up (data available on request).
Table 1.
Clinicodemographic characteristics of patients with depression referred to psychiatric care (n = 128)
| Women n = 85 (%) |
Men n = 43 (%) |
Total, n = 128 (%) | p-value* | |
|---|---|---|---|---|
| Age (mean) | 38 | 39 | 38 | |
| Marital status | 0.130d | |||
| Married, in a registered partnership, cohabiting | 31 (36.5) | 10 (23.3) | 41 (32) | |
| Unmarried, separated, widowed | 54 (63.5) | 33 (76.7) | 87 (68) | |
| Guardian of minors a | 21 (24.7) | 6 (14) | 27 (21.1) | 0.150d |
| Housing type | ||||
| Living alone (tested for living alone vs. living with others) | 31 (36.5) | 22 (51.2) | 53 (41.4) | 0.111d |
| Living with immediate family | 51 (60) | 17 (39.5) | 68 (53.1) | |
| Homeless | 0 | 1 (2.3) | 1 (0.8) | |
| Other | 3 (3.5) | 3 (7) | 6 (4.7) | |
| Education (highest completed degree) | ||||
| Basic (no high school or vocational training) | 23 (27.1) | 20 (46.5) | 43 (33.6) | |
| Intermediate (high school or vocational school) | 26 (30.6) | 13 (30.2) | 39 (30.5) | |
| High (higher vocational school, polytechnic, or university) | 36 (42.4) | 10 (23.3) | 46 (35.9) | 0.047d |
| Work status | ||||
| Employed | 20 (23.5) | 10 (23.3) | 30 (23.4) | |
| Student | 17 (20) | 4 (9.3) | 21 (16.4) | |
| Unemployed | 5 (5.9) | 10 (23.3) | 15 (11.7) | |
| Retired, disability pension for medical reasons | 13 (10.2) | 6 (14) | 19 (14.8) | |
| Sick leave | 25 (29.4) | 11 (25.6) | 36 (28.1) | |
| Disability pension for psychiatric reasons (disability > 12 months) | 1 (1.2) | 1 (2.3) | 2 (1.6) | |
| Parental leave, military, or non-military service | 4 (4.7) | 1 (2.3) | 5 (3.9) | |
| Part of active workforce (including sick leave ≤ 12 months) | 70 (82.4) | 35 (81.4) | 105 (82) | < 0.001d |
| Own perceived working ability b | 0.281d | |||
| Good | 10 (11.8) | 9 (20.9) | 19 (14.8) | |
| Impaired | 37 (43.5) | 14 (32.6) | 51 (39.8) | |
| Unable to work | 29 (34.1) | 16 (37.2) | 45 (35.2) | |
| Own perceived financial status | 0.149d | |||
| Good, adequate | 49 (57.6) | 19 (44.2) | 68 (53.1) | |
| Fair, poor | 36 (42.4) | 24 (55.8) | 60 (46.9) | |
| Chronic medical conditions | 36 (42.4) | 25 (58.1) | 61 (47.7) | 0.091d |
| AUDIT ≥ 8 | 21 (24.7) | 9 (20.9) | 30 (23.4) | 0.227d |
| Regular smoking | 20 (23.5) | 9 (20.9) | 29 (22.7) | 0.110d |
| Illegal drug use within 12 months c | 6 (7.1) | 5 (11.6) | 11 (8.6) | 0.508e |
AUDIT, Alcohol Use Disorders Identification Test (scores ≥ 8 indicating harmful alcohol consumption)
*Men vs. women
aData missing for 1 woman (1.2%)
bData missing for 9 women (10.6%) and 4 men (9.3%)
cData missing for 4 women (4.7%) and 1 man (2.3%)
dChi-square test
eFisher exact test
Prior course of Illness
Most (n = 106; 82.8%) of the 128 patients were diagnosed with recurrent depression with a median of three episodes (Table 2). The median age for all patients’ first MDE was 17 years (range 6 to 83 years, mean 24 years). Patients had suffered from their current MDE for a median of 6.5 months, but 19.5% (n = 25) had a chronic index episode of two years or longer. Patients with recurrent depression reported a shorter duration of symptoms (median 5.5 months vs. 16.0 months, p = 0.005) and had sought treatment faster (median five months vs. six months, p = 0.048) than first-episode patients. First-episode depression was also associated with a likelihood of a chronic index episode (p = 0.014) but not with a more severe level of depression at baseline. TRD patients did not differ from others regarding the baseline severity of depression, anxiety symptoms, or functional impairment, nor were they associated with chronic medical problems (data available on request).
Table 2.
Preceding course of depression and clinical and treatment characteristics of psychiatric patients with depression (n = 128)
| Current ICD-10 diagnosis of depression for n = 128 | |
|---|---|
| - F32 (Depressive episode): n (%) | 22 (17.2) |
| - F33 (Recurrent depressive disorder): n (%) | 106 (82.8) |
| Age at first depressive episode (years): Median age for n = 128 (IQR)a | 17 (17) |
| - F32: Median age (IQR) | 21 (17) |
| - F33: Median age (IQR) | 16 (18) |
| Total number of depressive episodes for patients with recurrent depression (n = 106) | |
| - Number of depressive episodes: Median (IQR) | 3 (2) |
| - Reached full recovery after previous depressive episodeb: Median (IQR) | 85 (80.2) |
| Current depressive episode for n = 128 | |
| - Psychiatric caretaking institution at recruitment | |
| Outpatient clinic: n (%) | 113 (88.3) |
| Hospital ward: n (%) | 15 (11.7) |
| Time from first symptom onset (months): Median (IQR) | 14.5 (26.8) |
| - Time from onset of depressive episode (months): Median (IQR) | 6.5 (9.8) |
| - Time from first consultation (months): Median (IQR)c | 5 (10) |
| - Patients using ADs before referral to current treatment: n (%) | 91 (71.1) |
| - Patients with previous AD trials (n = 91) for their current depression episode | |
| 1 previous AD trial: n (%) | 46 (50.5) |
| 2 previous AD trials: n (%) | 25 (27.5) |
| 3–6 previous AD trials: n (%) | 20 (22) |
| - Psychotherapy received before referral to current treatment: n = 128 (%) | 27 (21.1) |
IQR, interquartile range; AD, antidepressant
aMissing data for 2/128 patients (1.6%)
bMissing data for 1/106 patients with recurrent depression (0.9%)
cMissing data for 1/128 patients (0.8%)
Treatment received for current depressive episode (index episode) before baseline
Ninety-one (71.1%) of the 128 patients had used ADs for their current episode before study participation, half of whom (n = 46; 50.5%) had only one AD, with no significant difference between recurrent and first-episode depression patient groups. Commonly used ADs were escitalopram (n = 43), bupropion (n = 23), mirtazapine (n = 21), and venlafaxine (n = 17). Among the 128 patients, 18.8% (n = 24) had not responded to at least two adequately conducted AD trials before entering the study and were identified as TRD patients.
Before baseline, 21.1% (n = 27) of the patients attended psychotherapy, and over half (n = 72; 56.3%) reported attending supportive discussions with a health care professional. However, 25% (n = 32) had no regular treatment contact for their current depression before referral to psychiatric care.
Treatment received during the study
We present an overview of the treatment received during follow-up in Table 3. In accordance with the inclusion criteria, all 128 study patients were prescribed an AD at baseline. The most prescribed ADs were escitalopram (n = 22), bupropion and venlafaxine (n = 18 each), duloxetine (n = 17), and sertraline (n = 16). At eight weeks, 90.7% (n = 97) of the 107 patients remaining in the follow-up reported currently using ADs, 93.8% (n = 91) of whom had a dosage within the therapeutic range recommended by the Finnish DCG. Thirty (30.9%) of the 97 patients using ADs used an AD combination. Quetiapine was the only atypical antipsychotic used for AD augmentation, prescribed to nine patients (9.3%), one of whom also received augmentation with lamotrigine.
Table 3.
Treatment of psychiatric patients with depressive disorders during an eight-week follow-up (n = 107)
| All patients n = 107 N (%)a |
TRD n = 20b N (%) |
non-TRD n = 87 N (%) |
p-value* | |
|---|---|---|---|---|
| Follow-up appointments | ||||
| - Psychiatrist (A) | 73 (68.2) | 13 (65) | 60 (69) | 0.963c |
| - Other health care worker (B) | 74 (69.2) | 13 (65) | 61 (70.1) | 0.884c |
| - Any appointment overall (A, B, or A + B) | 94 (87.9) | 17 (85) | 77 (88.5) | 1d |
| - No follow-up appointments | 12 (11.2) | 2 (10) | 10 (11.5) | |
| Psychotherapy and other treatment sessions | ||||
| - Psychotherapy | 14 (13.1) | 4 (20) | 10 (11.5) | |
| - Supportive meetings at another service provider | 64 (59.8) | 14 (70) | 50 (57.5) | |
| - Family meetings | 1 (0.9) | 0 | 1 (1.1) | |
| - Group counselling | 5 (4.7) | 0 | 5 (5.7) | |
| - Visits at occupational health care unit | 2 (1.9) | 1 (5) | 1 (1.1) | |
| Use of antidepressants (ADs) at eight weeks | ||||
| Patients using ADs (ntot for AD use): | 97 (90.7) | 18 (90) | 79 (90.8) | 1d |
| - using AD monotherapy only | 67 (69.1) | 14 (77.8) | 53 (67.1) | |
| - using a combination of ADs | 30 (30.9) | 4 (22.2) | 26 (32.9) | 0.439c |
| - AD dosage compatible with Finnish DCG recommendations for a therapeutic dose | 91 (93.8) | 18 (100) | 73 (92.4) | 0.298d |
| Did the patient continue using the index AD prescribed at BL until eight weeks? | ||||
| - Yes, used index AD as only AD | 50 (51.5) | 10 (55.6) | 40 (50.6) | |
| - Yes, used index AD in combination with other AD(s) | 27 (25.2) | 3 (16.7) | 24 (30.4) | |
| - No, index AD switched to another AD, which was used alone or combined with other AD(s) | 15 (15.5) | 4 (22.2) | 11 (13.2) | |
| - No, index AD terminated, patient continued using previously prescribed AD(s) | 5 (4.7) | 1 (5.6) | 4 (5.1) | |
| - No, all ADs terminated | 8 (7.5) | 1 (5.6) | 7 (8.9) | |
| - No, never started using the index AD and not using any other AD | 1 (0.9) | 0 | 1 (1.3) | |
| Augmentation pharmacotherapy (combined with ADs) | 1d | |||
| - Atypical antipsychotic (AA) (quetiapine, minimum 50 mg/day) | 9 (9.3) | 1 (5.6) | 8 (10.1) | 1d |
| - Mood stabilizer (lamotrigine) | 1 (1.0) | 0 | 1 (1.3) | |
| Monotherapy: using one AD or one AA only (quetiapine ≥ 50 mg/day) | 62 (58.0) | |||
| ECT during follow-up (n = 107) | 1 (0.9) | 0 | 1 (1.5) | |
| New hospitalization during follow-up (n = 107) | 2 (1.9) | 0 | 2 (2.3) | |
AD, antidepressant; index AD, antidepressant assigned to study patient at baseline; AA, atypical antipsychotic; ECT, electroconvulsive therapy; BL, baseline; TRD, patient classified with treatment-resistant depression at baseline; non-TRD, patient classified as not having treatment-resistant depression at baseline
aMissing data for 1/107 patients (0.9%)
bMissing data for 1/20 TRD patients (5%)
cChi-square test
dFisher exact test
*TRD vs. non-TRD
Of the 107 patients finishing the study, roughly 1/3 had no or only one follow-up visit, 1/3 had two visits, and 1/3 had more than two visits (median for all patients was 2 visits, range 0 to 24 visits). Psychiatrists met the study patients on average once during the follow-up, but 30.8% (n = 33) of patients did not meet their treating psychiatrist after the initial meeting. However, three patients had multiple follow-up visits (18, 20, and 24 visits), mainly with a psychiatric nurse, together constituting 17.2% of all follow-up visits among study patients. All three patients suffered recurrent depression, classified as severe for one, moderate for one, and mild for one. None of these three patients reported acute suicidality. The TRD and non-TRD patients did not differ in the frequency of follow-up visits.
Altogether 53.3% of all patients received overall treatment classified as “minimally adequate”, including adequate pharmacological treatment and follow-up. As the Finnish DCG recommendations differ for the TRD and non-TRD patients, we examined the adequacy of treatment received separately for these two patient groups.
Treatment of non-TRD patients
Eighty-seven non-TRD patients (83.7%) finished the study, at which point most (n = 79; 90.8%) used ADs. Of the 79 non-TRD patients using ADs, 92.4% (n = 73) had a DCG-concordant treatment dosage, 32.9% (n = 26) used an AD combination, and 10.1% (n = 8) received AD augmentation with quetiapine, one additionally with lamotrigine. Furthermore, one non-TRD patient received electroconvulsive therapy (ECT) during follow-up.
Accounting for suboptimal follow-up, while 83.9% (n = 73) of all non-TRD patients completing the study received DCG-recommended AD treatment, only 60.9% (n = 53) received overall treatment meeting our definition of “minimally adequate”.
Treatment of TRD patients
Four TRD patients dropped out of the study, resulting in 83.3% (n = 20) being evaluated at eight weeks. Most (n = 18; 90%) used ADs, all of whom had a DCG-concordant AD treatment dosage. However, 77.8% (n = 14/18) used AD monotherapy. Consequently, as only four TRD patients used AD combinations, and one received augmentation using quetiapine, this resulted in 25% (n = 5) of all TRD patients receiving DCG-recommended pharmacological treatment for depression. Compared with the non-TRD patients, a significantly smaller proportion received adequate pharmacotherapy for their depression (25% vs. 83.9%, p < 0.005).
Altogether 20% (n = 4) of the 20 TRD patients evaluated at eight weeks had received treatment meeting the criteria for “minimally adequate,” i.e., having a DCG-concordant AD dosage at eight weeks, using pharmacological augmentation or an AD combination, and having had two or more follow-up visits at the treating facility. Overall treatment adequacy was therefore significantly lower (p = 0.002) than in non-TRD patients.
Patient outcomes
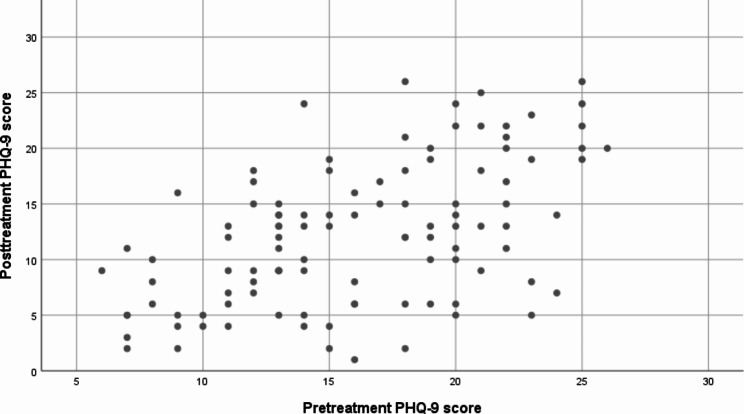
Our primary outcome was the within-individual change in PHQ-9 scores (Fig. 1). A significant difference (p < 0.0005) of 3.8 points emerged between PHQ-9 scores at baseline and eight weeks. At eight weeks, 10.3% (n = 11) of patients remaining in the study had a PHQ-9 score of ≤ 4 points, considered full remission, and 39.3% (n = 42) had a score of < 10 points, indicating subthreshold symptoms or remission.
Fig. 1.
Scatter plot of PHQ-9 scores before and after treatment in psychiatric patients with depressive disorders. PHQ-9, Patient Health Questionnaire
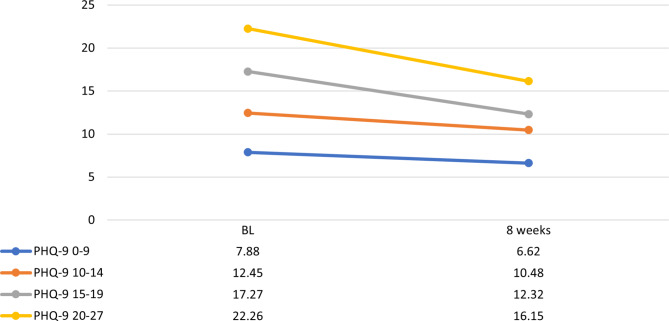
Self-report scale results are presented in Table 4. Mean PHQ-9 scores at baseline indicated moderately severe depressive symptoms (16 ± 5.1), decreasing to a mild level (12.2 ± 6.3) over the eight-week follow-up (p = 0.005). However, when patients were divided into groups according to baseline depression severity, wide variations emerged. At baseline, 12.5% (n = 16) reported subthreshold depressive symptoms (PHQ-9 < 10), 31.3% (n = 40) reported mild symptoms (PHQ-9 10–14), 28.9% (n = 37) reported moderate symptoms (PHQ 15–19), and 27.3% (n = 35) reported severe symptoms (PHQ-9 ≥ 20). The PHQ-9 score change at eight weeks reached statistical significance for all baseline depression severity groups with a baseline PHQ-9 score ≥ 10 (Fig. 2).
Table 4.
Self-report scale results of psychiatric patients (n = 107) with depressive disorders at baseline and eight weeks
| Baseline, n = 128 Mean (SD) |
Eight weeks, n = 107 Mean (SD) |
Change (8 − 0 weeks) Mean (SD) |
p-value* | |
|---|---|---|---|---|
| PHQ-9 | 16.0 (5.1) | 12.2 (6.3) | -3.8 (5.7) | p < 0.0005 |
| Oasis | 12.0 (3.7) | 9.9 (4.4) | -2.0 (3.8) | p < 0.0005 |
| SDS | 21.1 (6) | 17.9 (7) | -3.4 (7.1) | p < 0.0005 |
PHQ-9, Patient Health Questionnaire; Oasis, Overall Anxiety and Impairment Scale; SDS, Sheehan Disability Scale; SD, Standard deviation
*Baseline vs. eight-week time point, Paired samples T-test
Fig. 2.
Change in PHQ-9 scores in psychiatric patients with depressive disorders. Group division based on baseline depression severity. Y-axis: PHQ-9 scores, X-axis: time points at baseline (BL) and 8 weeks. PHQ-9, Patient Health Questionnaire; PHQ-9 0–9, mild depression; PHQ-9 10–14, moderate depression; PHQ-9 15–19, moderately severe depression; PHQ-9 ≥ 20, severe depression
We ran a multivariate logistic regression model to ascertain the effects of eight independent variables (age, sex, baseline values of PHQ-9, OASIS, AUDIT, and MSI, the variables indicating either first episode or recurrent depression, and AD dosage as either adequate or not) on the likelihood of reaching a PHQ-9 value < 10. The model was statistically significant, X^2(8) = 31.080, p < 0.005, explaining 39.2% of the variance and correctly classifying 79.6% of the cases (sensitivity 62.5%, specificity 88.5%, PPV 74.1%, and NPV 81.8%). Among the independent variables, only three were statistically significant: baseline PHQ-9 (B=-0.251, p = 0.001), the variable marking first episode vs. recurrent depression (F32 or F33) (B = 2.904, p = 0.009), and sex (B=-1.436, p = 0.032). Better odds of reaching a subthreshold level of depression symptoms (PHQ-9 < 10) were associated with a lower PHQ-9 value at baseline (OR 0.778, 95% CI: 0.669–0.906), having a diagnosis of recurrent depression (OR 18.246, 95% CI: 2.038- 163.345), and being female (OR 0.238, 95% CI: 0.064–0.885).
Finally, we examined the effect of treatment adequacy on PHQ-9 score change for TRD and non-TRD patients separately. Despite some numerical differences, no significant PHQ-9 score reduction was seen in favour of adequate treatment in either patient group (p = 0.12 for TRD patients and p = 0.09 for non-TRD patients).
Discussion
This study examined current treatment practice of depression, focusing on AD use in adult psychiatry units of Helsinki University Hospital, Finland. At baseline, most study patients had recurrent, moderately severe depression, and 19% had TRD. Roughly one-third had not received any AD treatment for their current MDE before referral to psychiatric care. During the eight-week follow-up, half of all patients received treatment meeting our minimum requirements for medication and follow-up. However, only few TRD patients received DCG-recommended AD combinations or pharmacological augmentation. Despite reported symptom relief and a significant mean decline in PHQ-9 scores, individual score reductions indicated modest treatment outcomes.
Patient characteristics and treatment history
Our typical patient was an educated female in her late thirties, referred to psychiatric care for her third, moderately severe depressive episode. Most of our study patients were diagnosed with recurrent depression, 19% with TRD, and 20%, predominately first-episode patients, had a chronic index episode with depression lasting two years or longer.
The mean baseline level of depression was less severe than expected. Initially, all patients had a PHQ-9 score ≥ 10. However, the study screening and the baseline evaluation did not always coincide, resulting in treatment initiation before evaluation in some patients. Baseline symptom ratings may also have been affected by pharmacological treatment initiated shortly before referral, causing symptom reductions while waiting for access to specialized psychiatric care.
Comparing patient characteristics with previously reported findings of patients in Finnish psychiatric care settings [40, 54], we find broadly similar sex and age distributions and mean severity of depression. We also see noteworthy differences compared with these previous studies. First, our patients seemed to suffer from a more persistent course of depression. Our study patients had their first depressive episode at a younger age, suffered more likely from recurrent depression, and had a longer symptomatic period of their current MDE before the baseline interview. Second, prior AD use was rarer than in Vuorilehto et al. [40], as nearly one-third of patients had not used any ADs for their current MDE before referral to psychiatric care.
Our finding of prolonged delay in referral to psychiatric care could reflect Finnish DCG recommendations of staging between different levels of care settings. However, it does not justify the lack of treatment trials in primary care. We did not find an explanation, such as a more severe mean level of depression or acute suicidality, for why so many patients were referred to psychiatric care without prior AD treatment. We do not know whether the failure to meet DCG recommendations is related to their not being appropriately conveyed, or to a scarcity of health care resources. We found some patient-related characteristics possibly influencing help-seeking behaviour, e.g., housing type (living alone vs. others) and lower employment rate, compared with earlier findings [40, 54].
While expecting a higher proportion of TRD among patients in psychiatric care, 19% is still somewhat higher than the 11% suggested in a Finnish nationwide register cohort [41], and it is within the range of prevalences found in other recent studies (e.g., [55–59]). Also, as some patients failed to provide information on AD dosing or duration, affecting the identification of prior adequate treatment trials, the actual proportion of TRD may have been higher. Hence, our ratio of TRD patients indicates that those deemed challenging to treat in primary care are at least partly correctly referred to psychiatric care.
Treatment during follow-up
Depression chronicity and recurrence increase the probability of poor outcomes, both acute and long-term [60–64]. Therefore, characteristics associated with poor outcomes, namely TRD, should alert clinicians to consider treatment enhancement. However, our findings indicate that this may have been overlooked in our study patients’ treatment planning and follow-up.
The results regarding mere AD use or the bare minimum of follow-up visits appear acceptable. However, due to non-implemented DCG-recommended AD combination and augmentation strategies, the observed pharmacological treatment of TRD patients can be considered substandard. Follow-up visits were unevenly distributed among patients. One-third did not meet their psychiatrist in follow-up, yet a few patients received disproportionally many visits overall, which was not explained by depression severity or acute suicidality. Half of all patients and only 20% of TRD patients received treatment considered “minimally adequate”, meaning adequately combined pharmacological treatment and follow-up.
Our findings of current depression treatment indicate a clear gap between guideline recommendations and clinical practice but resonate with earlier results [27, 36, 41, 65]. Nevertheless, the now-observed quality-of-care problems warrant attention. Concerning treatment augmentation strategies, the DCG presents AD combinations or pharmacological augmentation as plausible options when treating TRD in psychiatric care. However, as patients are referred from primary to psychiatric care in severe situations requiring expertise, we consider the expectation of using AD combinations or pharmacological augmentation in treating TRD justified. Although some TRD patients may benefit from additional AD monotherapy trials [66], medication augmentation strategies are recommended to enhance outcomes [67–71]. In our study, AD combinations were equally likely to be administered to TRD and non-TRD patients, and augmentation pharmacotherapy was strikingly sparse in both patient groups. We do not know whether symptom rating scales were used during each visit to guide clinicians’ treatment decisions, but clearly, TRD patients did not receive DCG-recommended drug enhancement as a rule. Simply put, a similar treatment regimen was used for all patients, regardless of their medical history.
Explanations for DCG-discordant treatment practice might include unawareness of patients’ symptom and treatment history and, therefore, failure to recognize TRD. Staff shortages may affect the quality of care, and the clinical supervision of doctors in training, decreasing the awareness of DCG recommendations in primary and psychiatric care. Also, patients may be wary of using multiple pharmaceuticals or substances not primarily used for MDD for fear of side effects and stigmatization. However, patients and caregivers must be educated to recognize the risks of prolonged ineffective treatment.
Therefore, proper psychoeducation should not be overlooked as a stepping stone in depression treatment. We need to share information with patients on disabilities and poor outcomes connected to depression chronicity and recurrence, justifying enhanced treatment options when considered necessary.
Patient outcomes
Overall, symptom rating scores indicated significant improvement in all measured entities. However, measured by PHQ-9 score reductions, only 10% of patients reached depression remission and 30% treatment response. Notably, nearly half of the patients experienced no treatment response. These findings of suboptimal treatment outcomes cannot be overlooked despite the relatively short study duration of eight weeks and the uncertainty of effective dose adjustment for a maximum response during follow-up.
The finding of a substantial proportion of non-responders may relate to clinical characteristics, such as depression chronicity in the form of a longer symptomatic period, evident at baseline. Chronicity was seen notably in the first-episode patients. In contrast, patients with recurrent depression showed faster initiation of treatment and better treatment results. Our result suggests an accentuation of chronicity compared with earlier findings from a Finnish psychiatric care cohort [54]. In this earlier study, treatment response was mainly seen within six months of treatment initiation in psychiatric care, after which recovery was sparse. Other studies also stress the effectiveness of treatment early in illness [63, 72], that a greater illness burden reflects on the treatment required, and that results diminish for each treatment step needed [60, 66].
We observed PHQ-9 scores decreasing more between baseline and the two-week time point than in the last six weeks of follow-up. The reason was not clarified but may relate to an unspecified reaction to treatment initiation. However, as early treatment response has been associated with better outcomes [16, 73], the possible predictive nature of this clinical factor should be noted in follow-up. Nevertheless, this finding highlights the importance of repeated symptom and response tracking throughout the first weeks and months of acute depression treatment to ensure treatment intensification if needed.
Study strengths and limitations
This study aimed at a representative sample of patients with depressive disorders referred to psychiatric care with a PHQ-9 score ≥ 10 and a new AD initiated. Detailed data were collected during an eight-week prospective study using in-person interviews and standardized evaluation scales. This study highlights the flow of patients from primary to specialized psychiatric care and follows patients in usual treatment. Therefore, we describe current, real-life patient characteristics and depression treatment strategies used in psychiatric care.
There are some limitations to consider. First, the number of patients recruited was moderate (n = 131). Second, we did not collect data on the number of patients declining participation or the reason for this, both limitations affecting the generalizability of the results. However, given the similarity of characteristics between our cohort and other comparable psychiatric cohorts within the Helsinki University Hospital area [40, 54], we find it unlikely that this would have resulted in a marked selection bias concerning the AD treatment provided to our study patients. In our view, possible selection bias may have enriched adherent doctors and patients, and patients without characteristics necessitating urgent clinical measures such as imminent suicide risk. Also, similar patient characteristics and suboptimal treatment practices have been reported in a recent nationwide register-based cohort study [41], further supporting our findings. Third, data collection on the prior course of illness and treatment received relied on patients’ recollections, a source for possible data inaccuracy. For example, missing data on AD dosing and duration of use affected the recognition of adequate treatment trials, likely resulting in a slight underestimation of TRD prevalence. Fourth, despite longitudinal follow-up, data collection focused on the current cross-sectional state of two and eight weeks, limiting the information available on daily medication adherence, planned and realized dose changes between research time points. Therefore, even if meeting the therapeutic dose criteria of the DCG, the AD dosage could be suboptimal at an individual level. Finally, we also compared outcomes between patients with adequate vs. inadequate treatment and found no significant difference. However, this was a non-randomized, observational study from which causal inferences from the role of treatment on the observed outcome are not justified.
Conclusion
Our results suggest that inadequate treatment of depression continues to occur in psychiatric care settings. Observed treatment outcomes were modest, and only 10% of patients reached remission. AD treatment was lacking particularly for TRD patients, as only 25% received DCG-recommended AD combinations or pharmacological augmentation. The intensity of treatment monitoring was inadequate for one-third of all patients.
Considering the escalating health burden caused by depressive disorders impacting the economy and personal loss, these findings of suboptimal treatment practices warrant attention. Based on our findings, we stress the importance of structured data collection and use of MBC to ensure quality of all treatment for depression.
Acknowledgements
We would like to thank Drs. Ritva Arajärvi, Hannu Koponen, Katariina Lönnfors and Kari Raaska plus Lic. Psychol Petri Näätänen for their help in collecting the PEGAD data, and professors Janne Backman, Mikko Niemi and Tiina Paunio for their contributions to the PEGAD study design.
Abbreviations
- AD
Antidepressant
- AUDIT
The Alcohol Use Disorders Identification Test
- C-SSRS
The Columbia–Suicide Severity Rating Scale
- DCG
Depression care guideline
- FTND
The Fagerström Test for Nicotine Dependence
- ICD-10
International Statistical Classification of Diseases and Related Health Problems 10th Revision
- MBC
Measurement-based care
- MDD
Major depressive disorder
- MDE
Major depressive episode
- MDQ
The Mood Disorder Scale
- MSI-BPD
The McLean Screening Instrument for Borderline Personality Disorder
- OASIS
The Overall Anxiety and Impairment Scale
- PHQ-9
The Patient Health Questionnaire-9
- SDS
The Sheehan Disability Scale
- SHAPS
The Snaith-Hamilton Pleasure scale
- TRD
Treatment resistant depression
Authors’ contributions
EI conceptualized the study design and data collection and is the PI of the study. JvK and IB analyzed and interpreted the patient data. JvK drafted the manuscript, to which IB and EI provided critical revisions and gave valuable input on the interpretation of results.PJ, TT and MH participated in collecting data. All authors have critically commented and approved the final manuscript.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
The PEGAD was funded by a grant (TYH2018307) from the Helsinki University Hospital.
Data Availability
The datasets generated and analysed during the current study are not publicly available due to restrictions imposed by the Finnish data protection legislation and research permits but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committee of Helsinki University Hospital and the Finnish Medicines Agency (FIMEA) approved the study protocol in 2018. All recruited patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
Dr. Heikkinen has participated as a researcher in clinical trials of mood disorder pharmacotherapies sponsored by Janssen. The other authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Association AP, APA. (2010) Practice guidelines for the treatment of patients with major depressive disorder. Am Psychiatric Association. http://www.psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf.
- 2.Kennedy SH, Lam RW, Mcintyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder. Can J Psychiatry. 2016;61(9):540–60. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malhi GS, Bell E, Bassett D, Boyce P, Bryant R, Hazell P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Australian & New Zealand Journal of Psychiatry. 2021;55(1):7–117. doi: 10.1177/0004867420979353. [DOI] [PubMed] [Google Scholar]
- 4.[NG222] Ng. Depression in adults: treatment and management [Available from: https://www.nice.org.uk/guidance/ng222. [PubMed]
- 5.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant Drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. The Lancet. 2018;391(10128):1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duodecim TFMS, Working group set up by the Finnish Medical Society Duodecim and the Finnish Psychiatric Association. Depression. Current Care Guidelines. Helsinki: The Finnish Medical Society Duodecim, 2022 (referred 13.2.2023). Available online at: www.kaypahoito.fi.
- 7.Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, et al. Defining treatment‐resistant Depression Depression and Anxiety. 2020;37(2):134–45. doi: 10.1002/da.22968. [DOI] [PubMed] [Google Scholar]
- 8.Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75(1):57–66. doi: 10.3949/ccjm.75.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Gaynes BN, Dusetzina SB, Ellis AR, Hansen RA, Farley JF, Miller WC, et al. Treating Depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114–9. doi: 10.1097/JCP.0b013e31823f705d. [DOI] [PubMed] [Google Scholar]
- 10.Henssler J, Alexander D, Schwarzer G, Bschor T, Baethge C. Combining antidepressants vs antidepressant monotherapy for treatment of patients with Acute Depression. JAMA Psychiatry. 2022;79(4):300–12. doi: 10.1001/jamapsychiatry.2021.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, Sarangi SC, Maiti R, Sood M, Reeta K. Efficacy and safety of adjunctive serotonin-dopamine activity modulators in Major Depression: a Meta‐Analysis of Randomized controlled trials. J Clin Pharmacol. 2022;62(6):721–32. doi: 10.1002/jcph.2022. [DOI] [PubMed] [Google Scholar]
- 12.Scott F, Hampsey E, Gnanapragasam S, Carter B, Marwood L, Taylor RW et al. Systematic review and meta-analysis of augmentation and combination treatments for early-stage treatment-resistant depression. J Psychopharmacol. 2022;2698811221104058. [DOI] [PMC free article] [PubMed]
- 13.Taylor RW, Marwood L, Oprea E, DeAngel V, Mather S, Valentini B, et al. Pharmacological augmentation in Unipolar Depression: a guide to the guidelines. Int J Neuropsychopharmacol. 2020;23(9):587–625. doi: 10.1093/ijnp/pyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vázquez GH, Bahji A, Undurraga J, Tondo L, Baldessarini RJ. Efficacy and tolerability of combination treatments for Major Depression: antidepressants plus Second-Generation antipsychotics vs. esketamine vs. Lithium J Psychopharmacol. 2021;35(8):890–900. doi: 10.1177/02698811211013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Carlo V, Calati R, Serretti A. Socio-demographic and clinical predictors of non-response/non-remission in treatment resistant depressed patients: a systematic review. Psychiatry Res. 2016;240:421–30. doi: 10.1016/j.psychres.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Perlman K, Benrimoh D, Israel S, Rollins C, Brown E, Tunteng J-F, et al. A systematic meta-review of predictors of antidepressant treatment outcome in major depressive disorder. J Affect Disord. 2019;243:503–15. doi: 10.1016/j.jad.2018.09.067. [DOI] [PubMed] [Google Scholar]
- 17.Simon GE, Perlis RH. Personalized Medicine for Depression: can we Match patients with treatments? Am J Psychiatry. 2010;167(12):1445–55. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivedi MH. Tools and strategies for Ongoing Assessment of Depression. J Clin Psychiatry. 2009;70(suppl 6):26–31. doi: 10.4088/JCP.8133su1c.04. [DOI] [PubMed] [Google Scholar]
- 19.Xiao L, Qi H, Zheng W, Xiang Y-T, Carmody TJ, Mayes TL, et al. The effectiveness of enhanced evidence-based care for depressive disorders: a meta-analysis of randomized controlled trials. Translational Psychiatry. 2021;11(1):531. doi: 10.1038/s41398-021-01638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu M, Hong RH, Yang T, Yang X, Wang X, Liu J, et al. The efficacy of measurement-based Care for Depressive disorders. J Clin Psychiatry. 2021;82(5):21r14034. doi: 10.4088/JCP.21r14034. [DOI] [PubMed] [Google Scholar]
- 21.Guo T, Xiang Y-T, Xiao L, Hu C-Q, Chiu HFK, Ungvari GS, et al. Measurement-based Care Versus Standard Care for Major Depression: a Randomized Controlled Trial with Blind Raters. Am J Psychiatry. 2015;172(10):1004–13. doi: 10.1176/appi.ajp.2015.14050652. [DOI] [PubMed] [Google Scholar]
- 22.Duhoux A, Fournier L, Nguyen CT, Roberge P, Beveridge R. Guideline concordance of treatment for depressive disorders in Canada. Soc Psychiatry Psychiatr Epidemiol. 2009;44(5):385–92. doi: 10.1007/s00127-008-0444-8. [DOI] [PubMed] [Google Scholar]
- 23.Fernández A, Haro Jm Fau - Codony M, Codony M, Fau - Vilagut G, Vilagut G, Fau - Martínez-Alonso M, Martínez-Alonso MF, Autonell J, Autonell JF, Salvador-Carulla L, et al. Treatment adequacy of anxiety and depressive disorders: primary versus specialised care in Spain. J Affect Disord. 2006;96(1–2):9–20. doi: 10.1016/j.jad.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder. JAMA. 2003;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 25.Lisinski A, Hieronymus F, Eriksson E, Wallerstedt SM. Low SSRI dosing in clinical practice—a register-based longitudinal study. Acta Psychiatrica Scandinavica. 2021;143(5):434–43. doi: 10.1111/acps.13275. [DOI] [PubMed] [Google Scholar]
- 26.Schneider F, Kratz S, Bermejo I, Menke R, Mulert C, Hegerl U, et al. Insufficient depression treatment in outpatient settings. Ger Med Sci. 2004;2:Doc01. [PMC free article] [PubMed] [Google Scholar]
- 27.Simon GE, Von Korff M, Rutter CM, Peterson DA. Treatment process and outcomes for Managed Care patients receiving New Antidepressant prescriptions from psychiatrists and Primary Care Physicians. Arch Gen Psychiatry. 2001;58(4):395–401. doi: 10.1001/archpsyc.58.4.395. [DOI] [PubMed] [Google Scholar]
- 28.Thornicroft G, Chatterji S, Evans-Lacko S, Gruber M, Sampson N, Aguilar-Gaxiola S, et al. Undertreatment of people with major depressive disorder in 21 countries. Br J Psychiatry. 2017;210(2):119–24. doi: 10.1192/bjp.bp.116.188078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vigo D, Haro JM, Hwang I, Aguilar-Gaxiola S, Alonso J, Borges G, et al. Toward measuring effective treatment coverage: critical bottlenecks in quality- and user-adjusted coverage for major depressive disorder. Psychol Med. 2022;52(10):1948–58. doi: 10.1017/S0033291720003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mojtabai R, Amin-Esmaeili M, Spivak S, Olfson M. Remission and treatment augmentation of Depression in the United States. J Clin Psychiatry. 2021;82(6):21m13988. doi: 10.4088/JCP.21m13988. [DOI] [PubMed] [Google Scholar]
- 31.Herzog DP, Wagner S, Ruckes C, Tadic A, Roll SC, Härter M, et al. Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: a naturalistic study. Eur Arch Psychiatry Clin NeuroSci. 2017;267(8):711–21. doi: 10.1007/s00406-017-0798-6. [DOI] [PubMed] [Google Scholar]
- 32.Gaynes BN, Rush AJ, Trivedi M, Wisniewski SR, Balasubramani GK, Spencer DC, et al. A direct comparison of presenting characteristics of depressed outpatients from primary vs. specialty care settings: preliminary findings from the STAR*D clinical trial. Gen Hosp Psychiatry. 2005;27(2):87–96. doi: 10.1016/j.genhosppsych.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Balasubramani GK, Spencer DC, et al. Major depression symptoms in Primary Care and Psychiatric Care settings: a cross-sectional analysis. The Annals of Family Medicine. 2007;5(2):126–34. doi: 10.1370/afm.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro-Rodríguez JI, Olariu E, Garnier-Lacueva C, Martín-López LM, Pérez-Solà V, Alonso J, et al. Diagnostic accuracy and adequacy of treatment of depressive and anxiety disorders: a comparison of primary care and specialized care patients. J Affect Disord. 2015;172:462–71. doi: 10.1016/j.jad.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 35.Gabilondo A, Rojas-Farreras S, Rodráguez A, Ferníndez A, Pinto-Meza A, Vilagut G, et al. Use of primary and specialized Mental Health Care for a major depressive episode in Spain by ESEMeD respondents. Psychiatric Serv. 2011;62(2):152–61. doi: 10.1176/ps.62.2.pss6202_0152. [DOI] [PubMed] [Google Scholar]
- 36.Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-Month Use of Mental Health Services in the United States. Arch Gen Psychiatry. 2005;62(6):629–40. doi: 10.1001/archpsyc.62.6.629. [DOI] [PubMed] [Google Scholar]
- 37.Kasteenpohja T, Marttunen M, Aalto-Setälä T, Perälä J, Saarni SI, Suvisaari J. Treatment received and treatment adequacy of depressive disorders among young adults in Finland. BMC Psychiatry. 2015;15(1):47. doi: 10.1186/s12888-015-0427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melartin TK, Rytsälä HJ, Leskelä US, Lestelä-Mielonen PS, Sokero TP, Isometsä ET. Continuity is the main challenge in treating major depressive disorder in psychiatric care. J Clin Psychiatry. 2005;66:220–7. doi: 10.4088/JCP.v66n0210. [DOI] [PubMed] [Google Scholar]
- 39.Rytsälä HJ, Melartin MD, Leskelä TKMD, US MA, Lestelä-Mielonen PS, Sokero MAT, Isometsä PMD. ET, M.D., Ph.D. A Record-Based Analysis of 803 Patients Treated for Depression in Psychiatric Care. J Clin Psychiatry. 2001;62:701-6. [DOI] [PubMed]
- 40.Vuorilehto MS, Melartin TK, Rytsälä HJ, Isometsä ET. Do characteristics of patients with major depressive disorder differ between primary and psychiatric care? Psychol Med. 2007;37(6):893–904. doi: 10.1017/S0033291707000098. [DOI] [PubMed] [Google Scholar]
- 41.Lähteenvuo M, Taipale H, Tanskanen A, Rannanpää S, Tiihonen J. Courses of treatment and risk factors for treatment-resistant depression in Finnish primary and special healthcare: a nationwide cohort study. J Affect Disord. 2022;308:236–42. doi: 10.1016/j.jad.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Linnaranta O. Selvitys psykiatristen sairaalapaikkojen määrästä. Terveyden ja hyvinvoinnin laitos THL; 2022. https://urn.fi/URN:ISBN:978-952-343-820-0.
- 43.WHO. World Health Organization . International classification of Disease. 10. Geneva: WHO; 1992. [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia–Suicide severity rating scale: initial validity and internal consistency findings from three Multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norman SB, Hami Cissell S, Means-Christensen AJ, Stein MB. Development and validation of an overall anxiety severity and impairment scale (OASIS) Depress Anxiety. 2006;23(4):245–9. doi: 10.1002/da.20182. [DOI] [PubMed] [Google Scholar]
- 47.Hirschfeld RMA. The Mood Disorder Questionnaire. The primary care companion to the. J Clin Psychiatry. 2002;04(01):9–11. doi: 10.4088/pcc.v04n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the Assessment of Hedonic Tone the Snaith–Hamilton pleasure scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan disability scale. Int Clin Psychopharmacol. 2008;23(2):70–83. doi: 10.1097/YIC.0b013e3282f2b4d6. [DOI] [PubMed] [Google Scholar]
- 50.de Meneses-Gaya C, Zuardi AW, Loureiro SR, Crippa JAS. Alcohol Use Disorders Identification Test (AUDIT): an updated systematic review of psychometric properties. Psychol Neurosci. 2009;2:83–97. doi: 10.3922/j.psns.2009.1.12. [DOI] [Google Scholar]
- 51.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Addiction. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 52.Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, Hennen J. A screening measure for BPD: the Mclean Screening Instrument for Borderline personality disorder (MSI-BPD) J Personal Disord. 2003;17(6):568–73. doi: 10.1521/pedi.17.6.568.25355. [DOI] [PubMed] [Google Scholar]
- 53.Statistics IS, for Windows V, Corp I. (Released 2017). IBM SPSS statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. In.
- 54.Melartin TK, Rytsälä HJ, Leskelä US, Lestelä-Mielonen PS, Sokero TP, Isometsä ET. Severity and comorbidity predict episode duration and recurrence of DSM-IV major depressive disorder. J Clin Psychiatry. 2004;65(6):810–9. doi: 10.4088/JCP.v65n0612. [DOI] [PubMed] [Google Scholar]
- 55.Fife D, Feng Y, Wang MY-H, Chang C-J, Liu C-Y, Juang H-T, et al. Epidemiology of pharmaceutically treated depression and treatment resistant depression in Taiwan. Psychiatry Res. 2017;252:277–83. doi: 10.1016/j.psychres.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Gronemann FH, Jorgensen MB, Nordentoft M, Andersen PK, Osler M. Incidence of, risk factors for, and Changes over Time in Treatment-Resistant Depression in Denmark: a Register-based Cohort Study. J Clin Psychiatry. 2018;79(4):17m11845. doi: 10.4088/JCP.17m11845. [DOI] [PubMed] [Google Scholar]
- 57.Hägg D, Brenner P, Reutfors J, Li G, Dibernardo A, Bodén R, et al. A register-based approach to identifying treatment-resistant depression—comparison with clinical definitions. PLoS ONE. 2020;15(7):e0236434. doi: 10.1371/journal.pone.0236434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahlich J, Tsukazawa S, Wiegand F. Estimating prevalence and Healthcare utilization for treatment-resistant depression in Japan: a retrospective claims database study. Drugs - Real World Outcomes. 2018;5(1):35–43. doi: 10.1007/s40801-017-0126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gronemann FH, Jorgensen MB, Nordentoft M, Andersen PK, Osler M. Treatment-resistant depression and risk of all-cause mortality and suicidality in Danish patients with major depression. J Affect Disord. 2021;287:204–13. doi: 10.1016/j.jad.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 60.Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord. 2009;116(1–2):4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Jonsson U, Bohman H, von Knorring L, Olsson G, Paaren A, von Knorring AL. Mental health outcome of long-term and episodic adolescent depression: 15-year follow-up of a community sample. J Affect Disord. 2011;130(3):395–404. doi: 10.1016/j.jad.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 62.Pettit JW, Lewinsohn PM, Roberts RE, Seeley JR, Monteith L. The long-term course of depression: development of an empirical index and identification of early adult outcomes. Psychol Med. 2009;39(3):403–12. doi: 10.1017/S0033291708003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rush AJ, Wisniewski SR, Zisook S, Fava M, Sung SC, Haley CL, et al. Is prior course of Illness relevant to acute or longer-term outcomes in depressed out-patients? A STAR*D report. Psychol Med. 2012;42(6):1131–49. doi: 10.1017/S0033291711002170. [DOI] [PubMed] [Google Scholar]
- 64.Satyanarayana S, Enns MW, Cox BJ, Sareen J. Prevalence and correlates of Chronic Depression in the Canadian Community Health Survey: Mental Health and Well-Being. Can J Psychiatry. 2009;54(6):389–98. doi: 10.1177/070674370905400606. [DOI] [PubMed] [Google Scholar]
- 65.Fernández A, Haro JM, Martinez-Alonso M, Demyttenaere K, Brugha TS, Autonell J, et al. Treatment adequacy for anxiety and depressive disorders in six European countries. Br J Psychiatry. 2007;190(2):172–3. doi: 10.1192/bjp.bp.106.023507. [DOI] [PubMed] [Google Scholar]
- 66.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 67.Bennabi D, Charpeaud T, Yrondi A, Genty J-B, Destouches S, Lancrenon S, et al. Clinical guidelines for the management of treatment-resistant depression: French recommendations from experts, the French Association for Biological Psychiatry and Neuropsychopharmacology and the fondation FondaMental. BMC Psychiatry. 2019;19(1):262. doi: 10.1186/s12888-019-2237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nuñez NA, Joseph B, Pahwa M, Kumar R, Resendez MG, Prokop LJ, et al. Augmentation strategies for treatment resistant major depression: a systematic review and network meta-analysis. J Affect Disord. 2022;302:385–400. doi: 10.1016/j.jad.2021.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strawbridge R, Carter B, Marwood L, Bandelow B, Tsapekos D, Nikolova VL, et al. Augmentation therapies for treatment-resistant depression: systematic review and meta-analysis. Br J Psychiatry. 2019;214(1):42–51. doi: 10.1192/bjp.2018.233. [DOI] [PubMed] [Google Scholar]
- 70.Zhou X, Keitner GI, Qin B, Ravindran AV, Bauer M, Del Giovane C, et al. Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network Meta-analysis. Int J Neuropsychopharmacol. 2015;18(11):pyv060. doi: 10.1093/ijnp/pyv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou X, Ravindran AV, Qin B, Del Giovane C, Li Q, Bauer M, et al. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry. 2015;76(4):e487–98. doi: 10.4088/JCP.14r09204. [DOI] [PubMed] [Google Scholar]
- 72.Kaymaz N, van Os J, Loonen AJ, Nolen WA. Evidence that patients with single versus recurrent depressive episodes are differentially sensitive to treatment discontinuation: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2008;69(9):1423–36. doi: 10.4088/JCP.v69n0910. [DOI] [PubMed] [Google Scholar]
- 73.Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70(3):344–53. doi: 10.4088/JCP.07m03780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to restrictions imposed by the Finnish data protection legislation and research permits but are available from the corresponding author on reasonable request.