Abstract
Background:
Declining muscle mass is not always accompanied by declining muscle strength in older adults, challenging the notion that low muscle mass is the sole criterion for diagnosing sarcopenia.
Objective:
This review aims to find out the relationships between muscle mass and muscle strength with physical performance in older adults.
Design:
This article was a systematic review using Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.
Data Sources and Methods:
We do a systematic search of observational studies that are published between 2013 and August 2023 in PubMed, ScienceDirect, Sage journal, Tripdatabase, Cochrane Library, Embase, and CINAHL. Two reviewers selected and extracted data independently and an association measure was recorded from included studies.
Results:
The review analyzed 17 observational studies conducted between 2013 and September 2023. The findings suggest that while declining muscle mass is often associated with sarcopenia, it may not always correspond to declining muscle strength in older individuals. The most common method used to measure muscle mass was bioelectrical impedance analysis, while handgrip strength was the predominant measure of muscle strength. Tests such as timed up and go and gait speed were used to assess physical performance.
Conclusions:
Physical performance in older adults is significantly related to muscle strength, whereas the relationship between muscle mass and physical performance is either weak or negligible. Therefore, when evaluating physical performance in older individuals, focusing on muscle strength is more important than muscle mass alone.
Keywords: Muscle mass, muscle strength, physical performance, older adults
Introduction
Muscle problems in older adults are a major concern that can cause a decline in physical function and quality of life. The aging process is associated with impaired mitochondrial function and disrupted pathways involved in muscle growth, contributing to muscle atrophy and the observed decline in muscle performance among older individuals. 1 Factors such as reduced physical activity, inadequate nutrition, and hormonal changes further contribute to neuromuscular junction insufficiency, compromised blood flow to muscles, decreased regenerative capacity due to a decline in muscle satellite cells, infiltration of inflammatory cells, and increased oxidative stress. These factors collectively disrupt the balance between muscle protein degradation and synthesis. 2 Consequently, the aging population experiences a loss of muscle mass and integrity, rendering them frail, dependent, and unable to carry out daily activities independently. 3
Physical performance is essential for older adults, as it has a substantial impact on their quality of life. Multiple factors, including muscle mass, muscle strength, genetics, the existence of chronic diseases, level of physical activity, mental wellness, and psychosocial factors, influence the physical performance of older adults.4–8 Muscle mass is vital to the physical performance of older adults. The age-related loss of muscle mass, also known as sarcopenia, is recognized as the primary element underlying the decline in physical performance among older adults. Sarcopenia leads to a decline in physical function, which, in turn, worsens the long-term challenges of independent home living. 9 Decreased muscle strength can also impair the functional capacities of older adults because muscle strength impacts nearly all daily activities, thereby increasing their activity dependence and dependency on others. In addition, current evidence shows that muscle strength influences physical performance more than muscle mass in older adults. 10
The Asian Working Group for Sarcopenia and the European Working Group on Sarcopenia in Older People guidelines have been pivotal in shaping the understanding and diagnosis of sarcopenia. Both guidelines have traditionally considered low muscle mass as the primary and mandatory criterion for sarcopenia diagnosis.11,12 This emphasis on muscle mass has been widely accepted and utilized in clinical practice. However, a divergence in thought emerged with the development of the Sarcopenia Definition and Outcomes Consortium (SDOC) and the Special Interest Group for Sarcopenia in the Elderly South Asian Working Action Group on SARCOpenia (SWAG-SARCO) guidelines. These newer guidelines challenged the conventional perspective by placing greater importance on low muscle strength as a diagnostic criterion for sarcopenia. According to SDOC and SWAG-SARCO, reduced muscle strength is a more critical factor than low muscle mass in assessing the functional impact of sarcopenia in older adults.13,14 This divide in the field of sarcopenia diagnosis has created a need for bridging the gap between the two sets of guidelines. Researchers and healthcare practitioners must explore the evidence and rationale behind each approach to develop a unified understanding of how muscle mass and muscle strength contribute to sarcopenia. By addressing this gap, we can pave the way for a more comprehensive and evidence-based approach to the diagnosis and management of sarcopenia in older individuals.
Declining muscle mass is not always parallel with declining muscle strength in older adults. Factors such as cellular, neural, metabolic changes, inflammation, and other unknown factors may contribute to the decline in muscle strength independently of muscle mass.15–17 A study by Hughes et al., 15 found that rates of decline in muscle strength were greater than the rates of decline in muscle mass. Strength declines were observed even in individuals who maintained or gained muscle mass over time. 15 Although there is a relationship between muscle mass and strength, they do not always exhibit a direct proportional correlation.18,19 In fact, certain studies have indicated that in older individuals, muscle weakness is primarily attributed to a decline in muscle quality rather than a loss of muscle mass. Several studies have shown that muscle quality, which refers to the ability of muscle fibers to generate force, plays an important role in age-related decline in muscle strength, independent of muscle mass.10,20 Additionally, changes in muscle fiber type and content may also contribute to age-related decline in muscle mass and strength. 21 The relationship between muscle mass, muscle strength, and physical performance in older adults is, however, the subject of relatively few reviews. This review aims to address the issue by integrating and analyzing prior studies about muscle mass and muscle strength on physical performance in older adults to provide new evidence on this subject.
Methods
A systematic search was carried out based on the guidelines from Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020. 22 Data were extracted, including measurements of muscle mass, muscle strength, and physical performance, from selected studies. Quantitative analyses involved assessing correlations, significance measures (e.g., p-values), and coefficient correlations to gauge the strength and statistical significance of relationships. These findings were then synthesized qualitatively, highlighting patterns and trends across studies.
Search strategy
A systematic search was performed on seven electronic databases from 2013 to September 2023: PubMed, ScienceDirect, Sage journal, Tripdatabase, Cochrane Library, Embase, and CINAHL. The keywords used are as follows: “muscle mass” OR “appendicular skeletal muscle index” OR “skeletal muscle index” OR “SMI” OR “APMI” OR “sarcopenia” AND “muscle strength” OR “muscle weakness” OR “handgrip strength” OR “knee extension strength” AND “physical performance” AND “elderly” OR “older adults” OR “geriatric”. For example, in PubMed, the initial search included terms such as “Muscle mass,” “Muscle strength,” “Physical performance,” and “Elderly” to ensure a wide coverage of potential articles. The search query was designed as follows: (“Muscle mass” OR “appendicular skeletal muscle index” OR “skeletal muscle index” OR “SMI” OR “APMI” OR “sarcopenia”) AND (“muscle strength” OR “muscle weakness” OR “handgrip strength” OR “knee extension strength”) AND (“Physical performance” OR “functional capacity”) AND (“Elderly” OR “older adults” OR “geriatric”).
Eligibility criteria
Observational studies or baseline data from Randomized Controlled Trials that were published between 2013 and September 2023 in English and Indonesian were included in this review. By concentrating on literature published from 2013, we aim to provide readers with the most current and up-to-date information. This focus on recent data enhances the relevance and applicability of our findings in the context of contemporary healthcare practices. Moreover, restricting the search to post-2013 literature helps us capture studies that adhere to more recent diagnostic criteria, which are likely to be more in line with current clinical practice. Studies with subjects less than 60 years old (older adult definition based on Indonesian Minister of Health Regulation No. 67 of 2015) 23 and studies that were not done on humans were excluded from this review. Using 60 years as the cut-off enhances the likelihood of obtaining a sufficient number of studies with relevant data, thereby ensuring the robustness and reliability of our systematic review.
Study selection and data extraction
Based on the study’s title and abstract, two reviewers independently searched and chose studies from each electronic database. The study that has been collected is imported to the Mendeley Reference Manager version 2.91. Any discrepancies were discussed with a third reviewer in order to reach a consensus. Data about the first author, title, demographics data (age, gender, study location), methodological data, and result data were extracted independently by two reviewers. The first author of the study was contacted to see if additional data was needed. Articles that did not directly measure or report on the relationships between muscle mass, muscle strength, and physical performance in the elderly population were excluded.
Risk of bias assessment
The Appraisal Tool for Cross-Sectional Studies (AXIS) is used to assess the risk of bias in this review because almost all the included studies used a cross-sectional study design (1 study uses a cohort design study). Each author carries out an independent assessment, then the results will be combined and discussed until an agreement is reached.
Quality assessment
The evidence quality for each outcome was evaluated using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) method. 24 Each author performs an independent assessment, and afterward, the results will be merged and deliberated upon until a consensus is established. The definition of evidence quality is defined in Table 1.
Table 1.
Definition of evidence quality.
| Category | Definition |
|---|---|
| High | Very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | Moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | Confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect |
| Very low | Very little confidence in the effect estimates: the true effect is likely to be substantially different from the estimate of effect |
Results
Study selection and characteristics
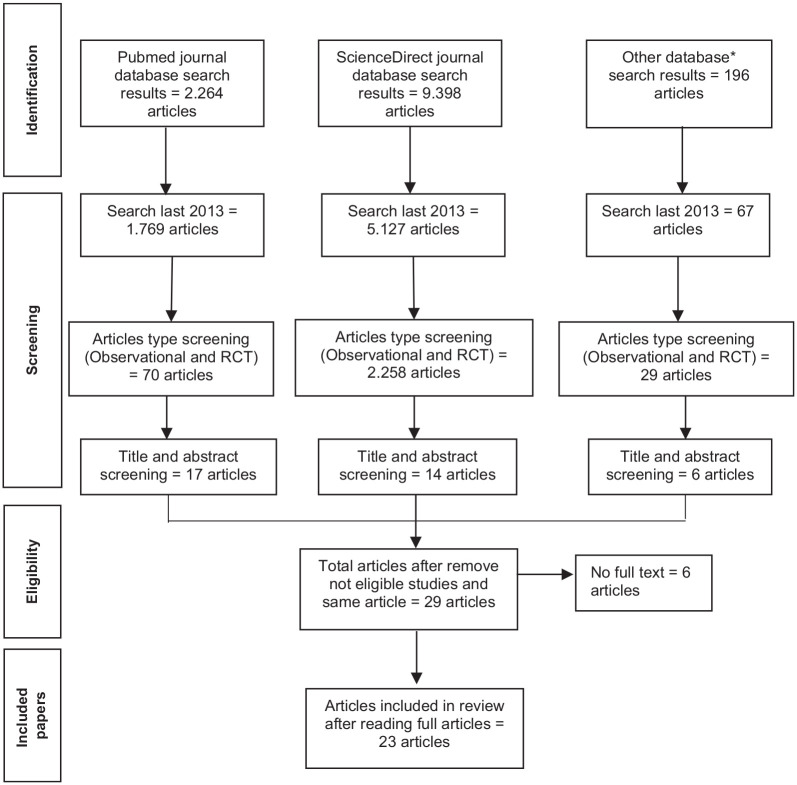
Following the initial search, a substantial number of articles were identified. For example, in the PubMed database, 2264 articles were retrieved in this step. To focus the review on observational studies, a filter specific to the study last 2013 was applied. This filtration process resulted in 1769 articles that met the criteria. After that, a filter for observational study types was employed, as denoted by the inclusion of the terms “Observational Study” and “Randomized Controlled Trial” (Publication Type). Out of the screening process, 70 articles were identified as observational studies and randomized controlled trials. The initial broad search was instrumental in capturing a wide range of relevant literature, while the subsequent application of the observational study filter ensured that the selected articles were deemed suitable for the systematic review’s objectives. After that, these 70 studies were carefully assessed and evaluated based on titles and abstracts simultaneously against the inclusion and exclusion criteria to determine the final set of studies included in the systematic review. Finally, 17 studies were captured from this database and all full texts were read to decide the final included studies. The search flowchart is shown in Figure 1.
Figure 1.
PRISMA flowchart.
A total of 37 articles were found in electronic databases which have relevant titles and abstracts to our eligibility criteria. After removing duplicate articles and reading full articles, 23 articles were finally included in this review. We excluded articles that have relevant titles but did not provide relationship measurement from the variable needed.25–34 The study selection process is shown in Figure 1. Cross-sectional design was used in eighteen studies, while cohort baseline data was used in four studies and RCT baseline data was used in one study. The studies were conducted on diverse regions, such as Japan (6 studies),19,35–39 Brazil (5 studies),40–44 Korea (3 studies),45–47 United States (2 studies),48,49 Indonesia (2 studies),50,51 and one study in Germany, 52 Poland, 53 Norway, 54 Spain, 55 and Peru. 56 A total of 3580 (male = 1108, female = 2472) subjects were recorded from the included studies. The range mean age of the subjects in this review was 64 years to 83.3 years.
This review examines different ways to measure muscle mass, strength, and physical performance in older adults. Bioelectrical impedance analysis (BIA) is the most widely used measurement tool in measuring muscle mass, namely 11 studies using this tool.19,36–39,42,45,50–53 Dual energy X-ray absorptiometry (DXA) which is also a recommended measurement tool used in six studies.43,44,46–49 While studies from Silva et al. 41 use calf circumference and Neves et al. 40 use a mathematical equation to measure muscle mass. Hand grip strength was the predominant measure of muscle strength, utilized in 19 out of the 23 studies incorporated in this review.35–42,44,45,47–53,55,56 Additionally, four studies employed knee extension strength as a measure of muscle strength.19,35,37,47,54 Various assessment methods were employed to evaluate physical performance across the studies included in this review. However, the gait speed (GS), chair stand test (CST), and time up and go (TUG) emerged as the most frequently used tests, employed in 14 studies,19,40,42,43,45,46,48–55 7 studies, and 6 studies respectively.
Risk of bias assessment
AXIS tool was used to assess the risk of bias in the included studies. Three studies indicate selection bias and three studies have insufficient information on sample selection. Regarding study limitations, three studies did not include limitations. Overall, three studies have a moderate risk of bias, while the others have a low risk of bias based on the author’s assessment. A summary of the risk of bias assessment is shown in Table 2.
Table 2.
Risk of bias assessment using AXIS.
| No. | Question | Study index number | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | ||
| 1 | Were the aims/objectives of the study clear? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2 | Was the study design appropriate for the stated aim(s)? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3 | Was the sample size justified? | Y | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | Y | UC | UC | UC | Y | UC | UC | UC | UC | UC | UC |
| 4 | Was the target/reference population clearly defined? (Is it clear who the research was about?) | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 5 | Was the sample frame taken from an appropriate population base so that it closely represented the target/reference population under investigation? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| 6 | Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation? | Y | Y | Y | Y | N | N | N | Y | Y | Y | UC | UC | Y | Y | Y | UC | Y | Y | Y | Y | Y | N | Y |
| 7 | Were measures undertaken to address and categorize non-responders? | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC |
| 8 | Were the risk factors and outcome variables measured appropriate to the aims of the study? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 9 | Were the risk factor and outcome variables measured correctly using instruments/measurements that had been trialed, piloted, or published previously? | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 10 | Is it clear what was used to determine statistical significance and/or precision estimates? (e.g., p-values, confidence intervals) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 11 | Were the methods (including statistical methods) sufficiently described to enable them to be repeated? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 12 | Were the basic data adequately described? | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 13 | Does the response rate raise concerns about non-response bias? | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 14 | If appropriate, was information about non-responders described? | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 15 | Were the results internally consistent? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 16 | Were the results presented for all the analyses described in the methods? | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 17 | Were the authors’ discussions and conclusions justified by the results? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 18 | Were the limitations of the study discussed? | Y | N | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| 19 | Were there any funding sources or conflicts of interest that may affect the authors’ interpretation of the results? | N | UC | N | N | N | N | N | N | UC | N | UC | N | N | N | N | UC | N | N | N | N | N | N | N |
| 20 | Was ethical approval or consent of participants attained? | Y | Y | Y | Y | Y | Y | Y | UC | Y | Y | Y | Y | Y | Y | UC | UC | Y | Y | Y | Y | Y | Y | Y |
| Overall results | L | M | M | L | L | L | M | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | |
UC: unclear; L: low; M: moderate; N: no; Y: yes.
Overall outcomes
Table 4 provides a summary of the study’s findings. In terms of the relationship between muscle mass and physical performance in older adults, 10 studies reported no significant association between these variables.19,36,40,42,44,47,48,51,53 However, nine studies indicated a significant relationship between muscle mass and physical performance, with one study having a moderate risk of bias. Specifically, one study reported a strong correlation, 52 two studies reported a moderate correlation41,45 and four studies reported a weak correlation.36,40,43,50 Overall, the quality of evidence for this relationship was graded as low, indicating inconsistent evidence to support the observed associations.
Table 4.
Summary of the results from included articles.
| Study index | Author | Sample detail | Study design | Measurement tools | Muscle mass and muscle strength | Muscle mass and physical performance | Muscle strength and physical performance | Subject condition |
|---|---|---|---|---|---|---|---|---|
| 1 | Neves et al. 30 | N: 387 (M = 141, F = 246), Mean age: M = 71 (65-93), F = 71 (65–90), Loc: Cuiaba, Brazil | Cross-sectional | SMM: Math—equations, MS: Handgrip dynamometer (Model SH5001, Saehan Corp., Masan, Korea), PP: Brazil version SPPB (GS, CST, balance test) | SMM has a significant relationship with HGS (p < 0.01, r = 0.625) | SMM does not have an association with CST (p > 0.05, r = 0.096), but has a significant association with GS (p < 0.01, r = 0.224) and balance (p < 0.05, r = 0.125) | Not available | Age ⩾ 65 years. Excluding elderly with DM, HT, using drugs affecting blood glucose, severe MR, Parkinson’s, amputation, and major orthopedic limitation |
| 2 | Elfa et al. 40 | N: 60 (M = 4, F = 56), Age: (n), 60–74 years = 53, ⩾75 years = 7, Loc: Banjarmasin, Indonesia | Cross-sectional | SMM: BIA Omron® HBF-375, MS: Jamar handgrip dynamometer, PP: TUG test and GS | Not available | SMM have significant correlation with TUG (p = 0.004, r = −0.342) and GS (p = 0.000, r = 0.491) | HGS have significant correlation with TUG (p = 0.015, r = −0.281) and GS (p = 0.000, r = 0.415) | Age > 60 years. Excluding elderly with uncorrectable vision disorder and obesity |
| 3 | Minematsu et al. 25 | N: 589 (M = 249, F = 340), Mean age: M = 73.7 ± 5.3, F = 73 ± 5.2, Loc: Nara, Japan | Cross-sectional | MS: HGS with a digital Hand grip dynamometer (TKK5401, Takei Scientific Instruments, Co., Ltd. Niigata, Japan); KES and KFS with isometric muscle strength dynamometer (μTasMF-01, ANIMA Corporation, Japan). PP: TUG, CST, 10MGT, FST, OLST, and MSL | Not available | Not available | Men with higher HGS performed significantly better in 10MGT, TUG, CRT, and OLST after adjusting for age and FST and MLS after adjusting for height and weight. A higher KES was associated with shorter CRT after adjusting for age and shorter FST after adjusting for height and weight. A higher KFS was associated with shorter 10MGT, TUG and CRT after adjusting for age, and additionally with shorter FST, higher OLST, and MLS, but not with 10MGT, after adjusting for height and weight. In women, higher muscle strength was significantly associated with better performance in all measures | Age ⩾ 65 years. Subjects were able to walk either with or without walking sticks and were able to communicate |
| 4 | Silva et al. 31 | N = 59 older women, Mean age: 71.5 ± 7 years, Loc: Campina Grande, Brazil | Cross-sectional | MM: Calf circumference with inelastic tape, MS: HGS with a manual hydraulic dynamometer (Dynamometer Takei Kiki Kogyo® TK 1201, Japan), PP: 30-s CST | MM has a significant correlation with MS in the physically active group (p = 0.013, r = 0.44) and physically inactive group (p = 0.022, r = 0.43) | MM has a significant correlation with PP in the physically active group (p = 0.015, r = −0.46) and physically inactive group (p = 0.004, r = 0.53) | Not available | Older women ⩾60 years, physically active and inactive. Any neurological disease or orthopedic limitation is excluded from this study |
| 5 | Iwase et al. 26 | N: 648 (M = 148, F = 536), Mean age: 73.2 ± 5.6, (M = 74.6 ± 5.1; F = 72.8 ± 5.6), Loc: Yasu, Japan | Cross-sectional | MM: BIA (InBody 430 (Biospace Co., Ltd., Seoul, Korea) and InBody 470 (InBody Japan Inc., Tokyo, Japan)), MS: HGS with a digital grip dynamometer (T.K.K.58401, Takei Scientific Instruments Co., Ltd., Niigata, Japan); QS with A handheld dynamometer (μTas F-1, Anima Corp., Tokyo, Japan). PP: TUG, OLST | In older men, both upper limb (p < 0.01, r = 0.43) and lower limb (p < 0.01, r = 0.36) MM has a significant correlation with HGS. Both upper limb (r = 0.05) and lower limb (r = 0.12) MM do not have a significant correlation with QS. In older women, both upper limb (p < 0.01, r = 0.64) and lower limb (p < 0.01, r = 0.54) MM has a significant correlation with HGS. Both upper limb (p < 0.01, r = 0.21) and lower limb (p < 0.01, r = 0.17) MM has significant correlation with QS | In older men, both upper limb and lower limb MM do not have a significant correlation with TUG and OLST. In older women, upper limb MM has a significant correlation with TUG (p < 0.05, r = −0.11) and OLST (p < 0.05, r = 0.11); lower limb MM has a significant correlation with TUG (p < 0.01, r = −0.16) and OLST (p < 0.05, r = 0.11) | Not available | Age ⩾ 65 years. Elderly with long-term care requirements, CNS disease, and MMSE ⩾24 are excluded from this study |
| 6 | Stoever et al. 42 | N: 71 older men, Mean age: 72.5 ± 3.9 years (65–83), Loc: Cologne Germany | Cross-sectional | MM: BIA EgoFit BIA series 4, mono frequency, Germany), MS: HGS with Jamar hand dynamometer, PP: SPPB (GS, CST, Balance test) | SMI has a significant correlation with HGS (p < 0.01, r = 0.555) | SMI has significant correlation with GS (p < 0.01, r = 0.724) and CST (p < 0.05, r = −0.283) | HGS has a significant correlation with GS (p < 0.01, r = 0.537), but does not have a significant correlation with CST (r = −0.206) | Age ⩾ 65 years. Serious inflammatory conditions, neurological disorders, orthopedic limitations, and heart disease were excluded from this study |
| 7 | Nishi et al. 28 | N: 30 older women, Age: 76.2 ± 7.3 years (65–92 years), Loc: Kobe, Japan | Cross-sectional | MM: BIA Inbody770 Japan Inc., Tokyo, Japan. MS: HGS with GRIP-D (Takei Ltd. Niigata, Japan); leg muscle strength with ALCARE Co., Ltd., Tokyo, Japan | Arm MM has a significant correlation with HGS (p < 0.001, r = 0.716), Leg MM does not have a significant correlation with leg muscle strength (p = 0.702, r = 0.082) | Not available | Not available | Age ⩾ 65 years. The elderly who can live independently and can communicate well were included in this study |
| 8 | Kristiana et al. 41 | N: 203 (M = 57, F = 146). Mean age: 64 years (60–86 years), Loc: Surabaya, Indonesia | Cross-sectional | MM: BIA, MS: HGS with hand dynamometer Takei Physical Fitness Test, PP: SPPB (balance test, GS, CST) | Not available | No correlation between MM and PP (p = 0.52) | HGS has a significant correlation with PP (p < 0.001, r = 0.26) | Age ⩾ 60 years. The elderly with ADL ⩽8, orthopedic limitation, arthritis, MMSE ⩽18, and heart disease were excluded from this study |
| 9 | JCM, de Oliveira et al. 32 | N: 53 (M = 6, F = 47). Mean age: non-fragile = 66 ± 6.1 years, fragile = 70 ± 5.2 years, Loc: Sao Paolo, Brazil |
Cross-sectional | MM: BIA tetrapolar, Device (Quantum II, RJL System, Clinton Twp, MI, USA), MS: HGS with a hand dynamometer, PP: GS | In the non-fragile group, MM has a significant correlation with HGS (p < 0.02, r = 0.7), while in the fragile group, the is no significant correlation (p < 0.10, r = 0.3) | In the fragile (p < 0.19, r = −0.2) and non-fragile (p < 0.39, r = −0.3) groups there is no significant correlation between MM and GS | Not available | Age ⩾ 60 years. Both fragile and non-fragile group |
| 10 | Irisawa et al. 29 | N: 179 (M = 90, F = 89), Age: 79.7 ± 11.5 years, Loc: Two stroke rehabilitation units in Japan | Cross-sectional | MM: BIA InBody S-10 analyzer (InBody Japan, Tokyo, Japan). MS: manual dynamometer (Grip A TKK5001, Takei Scientific Instruments Co. Ltd., Niigata, Japan). Functional: FIM motor score | Not available | MM has a weak correlation with functional status recovery in males (r = −0.14) and females (r = −0.22) | HGS has a significant correlation with functional status recovery in males (r = 0.66) and females (r = 0.45) | Stroke patients. Elderly with a pacemaker, severe cognitive impairment, severe dysphasia, FMI > 81, and early discharge were excluded from the study |
| 11 | Ossowski et al. 43 | N: 95 older women, Mean age: 68.76 ± 5.02 years (62–81 years), Loc: Gdansk, Polandia | Cross-sectional | MM: BIA InBody 720 analyzer. MS: HGS with a digital manual dynamometer (SAEHAN, Changwon, Korea). PP: CST, GS, SAC | Not available | Both SMM (p = 0.054, r = 0.21) and SMI (p = 0.92, r = 0.11) do not have a significant correlation with GS | HGS does not have a significant correlation with GS (p = 0.12, r = 0.18) | Age ⩾ 60 years. Postmenopausal women for at least 12 months |
| 12 | Hayasida et al. 19 | N: 318 (M = 111, F = 207), Mean age: M = 75.5 ± 5.5 years; F = 75.8 ± 6 years, Loc: Takatsuki, Japan | Cross-sectional | MM: BIA MC-190 (Tanita Corp., Tokyo, Japan), MS: KES with Handheld dynamometer (µTas-01, Anima Co., Tokyo, Japan), PP: GS | ASM has a significant correlation with KES both in men (p < 0.01, r = 0.34) and women (p < 0.01, r = 0.23) | ASM has no significant correlation with GS both in men (r = 0.11) and women (r = 0.07) | KES has a significant correlation with GS both in men (p < 0.01, r = 0.38) and women (p < 0.01, r = 0.45) | Age ⩾ 65 years, Community-Dwelling Elderly |
| 13 | Bardstu et al. 44 | N: 107 (M = 43, F = 63), Median age: 86 years (80–90), Loc: Norwegian | Cross-sectional | MS: KES with custom-made flexi-bench (Pivot 430 Flexi-bench, Sportsmaster, Norway) and a non-elastic band (ROPES A/S, Aasgardstrand, Norway) attached to a force cell (Ergotest Innovation AS, Langesund, Norway). PP: CST, TUG, GS | Not available | Not available | KES has significant correlation with 5-CST (p = 0.009), TUG (p < 0.001), GS (p < 0.001) | Age ⩾ 67 years. Community-dwelling elderly who got home care due to functional/medical disabilities |
| 14 | Nonaka et al. 27 | N: 192 elderly women, Mean age: 73.7 ± 5.8 years, Loc: Kyoto, Japan | Cross-sectional | MM: BIA InBody 430 (Biospace Co., Ltd. Seoul, Korea) for 107 subjects and 470 (InBody Japan Inc., Tokyo, Japan) for 85 subjects. MS: HGS with hand grip dynamometer (T.K.K.58401, Takei Scientific Instruments Co., Ltd., Niigata, Japan); KES with a handheld dynamometer (µTas F-1; Anima Corp., Tokyo, Japan) | Upper limb SMM has a significant association with HGS (p < 0.001), Lower limb SMM has a significant association with KES (p < 0.001) | Not available | Not available | Age ⩾ 60 years, Community-dwelling elderly women with no significant cognitive impairment |
| 15 | Duchowny et al. 38 | N: 40 old men, Mean age: 83.3 ± 3.9 years, Loc: United States | Cohort | MM: DXA scans (Hologic 4500 scanners, Waltham, MA), MS: Jamar handheld dynamometers, PP: GS | Change in SMI has no significant correlation with change in HGS (p = 0.109, r = 0.26) | Change in SMI has no significant correlation with change in GS (p = 0.604, r = -0.08) | Not available | Age > 65 years, Community-dwelling men without bilateral hip replacements |
| 16 | Kwon et al. 35 | N: 28 older women, Mean age: 69.9 ± 0.8 years, Loc: Seoul, Korea | RCT, (We only take cross-sectional data from this study) | MM: BIA, Inbody 720, Biospace, Korea, MS: HGS with digital hand dynamometer (Smedley, Takei Japan), PP: GS and TUG | SMM has significant correlation with HGS (p = 0.008, r = 0.49) | SMM has significant correlation with TUG (p = 0.04, r = -0.391) | HGS has significant correlation with GS (p = 0.037, r = 0.395) and TUG (p = 0.001, r = −0.614) | Age > 65 years, Subject from community welfare center |
| 17 | Palacios-Chavez et al. 46 | N: With diabetes = 139 (M = 43, F = 96); Without diabetes = 382 (M = 105, F = 277), Subject with diabetes age: 60–69 years: 82. 70–79 years: 50. ⩾80 years: 7, Subject without diabetes age: 60–69 years: 109, 70-79 years: 151, ⩾80 years: 122, Loc: Lima, Peru | Cross-sectional | MS: CAMRY dynamometer, PP: TUG | Not available | Not available | HGS has a significant association with TUG (p < 0.001) | Age > 60 years, Diabetes and non-diabetes patients |
| 18 | Falsarella et al. 33 | N: 99 old women (⩾65 years), Mean age: 77 ± 6.5 years, Loc: Brazil | Cross-sectional | MM: DXA, PP: TUG, GS | Not available | ASMI has significant correlation with TUG (r = 0.27, p = 0.006) | Not available | Community elderly |
| 19 | Kim et al. 37 | N: 560 older adults (⩾65 years), Loc: Korea | Baseline data from cohort study | MM: DXA, MS: HGS with a hand dynamometer (Hand Evaluation Kit; Sammons Preston, Bolingbrook, IL, USA), KES with an isokinetic dynamometer (Biodex Isokinetic Tester; Biodex Medical Systems, Shirley, NY, USA), PP: SPPB | Not available | ASMI has no significant association with physical performance (p = 0.86) | HGS has no association with PP (p = 0.93), but KES has a significant association with PP (p = 0.02) | Older resident in Sengnam, Korea |
| 20 | Hernandez-Luis et al. 45 | N: 298 hospitalized patients (⩾60 years), Mean age: 76.6 years, Loc: Spain | Baseline data from cohort study | MS: HGS with Collins dynamometer (Surtex-instruments, London, England, UK), PP: GS | Not available | Not available | HGS has significant correlation with GS (p = 0.000, r = 0.536) | Patients admitted for an acute illness or decompensation via the emergency room to the Internal Medicine Department of the Canary Islands University Hospital, Exclusion: terminal patients (with a life expectancy of <6 months) and patients with acute delirium or impaired consciousness that persisted on the second day of hospital admission |
| 21 | Shaffer et al. 39 | N: 1.112 (M: 536, F: 576) older adults (⩾60 years), Loc: United States | Cross-sectional | MM: DXA, MS: Grip/ASMI, PP: GS | Not available | ASM has a significant association with GS (p < 0.001) | Grip/ASMI does not have a significant association with GS (p > 0.05) | Healthy older adults |
| 22 | Vilaca et al. 34 | N: 75 elderly women (65–80 years), Mean age: 69.6 ± 4.4 years, Loc: Brazil | Cross-sectional | MM: DXA, MS: HGS with a hydraulic dynamometer (Saehan Corporation, Masan Free Trade Zone, Korea), PP: 6MWT | HGS has a significant association with arm lean mass (p < 0.01) | Lean mass does not have a significant association with 6MWT | Not available | Community-dwelling older women |
| 23 | Lee et al. 36 | N: 435 older adults (70–84 years), Loc: Korea | Baseline data from cohort study | MM: Lean mass with DXA, PP: GS | Not available | The lean mass of both legs has a significant association with GS (p = 0.03) | Not available | Community-dwelling older adults |
6MWT: 6-meter walking test; 10MGT: ten-meter gait time; AC: arm curl; ADL: activity daily living; ASM: appendicular skeletal mass; BIA: bioelectrical impedance analysis; CNS: central nervous system; CST: chair stand test; DM: diabetes melitus; DXA: dual energy X-ray absorptiometry; FST: floor standing time; GS: gait speed; HGS: handgrip strength; HT: hypertension; KES: knee extension strength; Loc: location; MM: muscle mass; MMSE: mini mental state examination; MS: muscle strength; MSL: maximum one step length to height ratio; OLST: one leg standing time; PP: physical performance; SPPB: short physical performance battery; SMI: skeletal muscle index; SMM: skeletal muscle mass; TUG: time up and go.
Conversely, in relation to muscle strength and physical performance in older adults, 10 out of 13 studies analyzing this relationship found a significant association between muscle strength and physical performance.19,35,39,45,50–52,54,56 Among these 10 studies, two reported a strong correlation,39,45 three reported a moderate correlation,19,50,52 one reported a weak correlation, 51 and the remaining four studies did not analyze the correlation coefficient.35,54–56 From these 10 studies, two studies have a moderate risk of bias. Only one study by Ossowski et al. 53 indicated no significant relationship between handgrip strength and GS. Consequently, the quality of evidence for the relationship between muscle strength and physical performance was rated as high, signifying a robust and dependable body of evidence supporting this connection.
In response to these results, we also conducted a study examining the relationship between muscle mass and muscle strength. A total of 10 out of 11 studies stated a significant relationship between muscle mass and muscle strength.19,36–38,40–42,45,52 On the other hand, according to the study conducted by Duchowny et al., 48 there was no statistically significant correlation observed between skeletal muscle index and hand grip strength. The quality of evidence for the link between muscle mass and muscle strength was also rated as high, indicating a solid foundation of evidence to support the observed associations. The GRADE quality assessments emphasize the significance of muscle strength and muscle mass in influencing physical performance among older people. A summary of evidence quality assessment is shown in Table 3.
Table 3.
Assessment of quality of the evidence.
| Assessed parameter | Results | Number of studies | Quality of the evidence (GRADE) |
|---|---|---|---|
| Association between muscle mass and physical performance in elderly | 9 studies show a significant association between muscle mass and physical performance (4 studies show a weak correlation, 2 studies show a moderate correlation and 1 study shows a strong correlation, while 10 studies show there’s no significant association between muscle mass and physical performance) | 17 Observational studies | Low |
| Association between muscle strength and physical performance in elderly | 10 studies show a significant association between muscle strength and physical performance with 2 studies showing a strong correlation, 4 studies showing a moderate correlation, 1 study showing a weak correlation, and 3 studies didn’t provide correlation analysis | 13 Observational studies | High |
| Association between muscle mass and muscle strength in elderly | 10 studies show a significant association between muscle mass and muscle strength with 3 studies showing a strong correlation, 4 studies showing a moderate correlation, 1 study showing a weak correlation, and 1 study did not provide correlation analysis | 11 Observational studies | High |
Discussion
Presently, the most recent consensus from both Asian and European working groups addresses the diagnosis of sarcopenia. In these consensuses, similar criteria are outlined, specifically involving a decline in muscle mass followed by a decrease in muscle strength and/or a reduction in physical performance.11,12 Nevertheless, numerous studies indicate that reduced muscle strength is linked to diminished physical performance and functional capacity in older individuals, irrespective of whether there is a concurrent decrease in muscle mass. Adhering to the current diagnostic criteria for sarcopenia may result in the underdiagnosis and delayed treatment of older adults who exhibit reduced muscle strength unaccompanied by a decrease in muscle mass, as these individuals would not be classified as having sarcopenia according to the existing criteria.
According to Macedo et al., 57 high levels of inflammatory mediators are predictors of a decline in functional capacity in older adults. This suggests that inflammation may play a role in the functional capacity of older adults, independent of muscle mass. Similar to other studies, muscle weakness in older adults is caused by both muscle atrophy and a decline in the quality of the muscle fibers. This shows that a significant component of an older adult’s functional capacity is muscle quality rather than merely muscle mass. 58 Older adults might perform physically better with exercise training. This shows that even in the face of decreasing muscle mass, physical activity can help maintain or increase physical performance. 59 Protein-rich diets and regular exercise can assist older adults in retaining muscle mass and increasing their functional capacity. This implies that maintaining muscle mass is crucial for preserving functional capacity, but that other elements like physical activity and diet also matter. 60 Additionally, according to the Baltimore Longitudinal Study on Aging Paradigm, mobility depends heavily on the strength of the muscles, and there is a significant discrepancy between muscle function and muscle mass. 61 This shows that measuring muscle mass alone may not be sufficient to predict functional decline in older people.
The association between muscle strength and physical performance in older adults has been extensively explored in scientific research. Declining muscle strength significantly contributes to diminished physical performance in older adults. Numerous studies have demonstrated that muscle strength exerts a more pronounced influence on physical performance than muscle mass in older individuals. 10 A study involving older adult individuals residing in the community in Japan revealed a strong relationship between muscle strength and maximal walking speed, whereas muscle mass did not exhibit a similar connection. These findings suggest that the preservation of muscle strength, rather than muscle mass, may hold greater importance in preserving mobility among the older adult population. 19 In line with a previous investigation conducted by Visser et al., 62 it has been noted that decreased muscle strength is associated with impaired physical performance. However, there is a scarcity of substantial empirical evidence supporting the correlation between muscle mass and physical function. The mechanism through which muscle strength influences physical performance and functional capacity can be attributed to several factors. Firstly, muscle strength is essential for generating force and power, which are required for activities such as walking, lifting objects, and maintaining balance.63,64 Adequate muscle strength allows individuals to perform these tasks efficiently and with reduced effort. Secondly, muscle strength is closely linked to muscle endurance, which is crucial for sustaining physical activity over time.63,64 Strong muscles are better able to withstand fatigue and maintain performance during prolonged activities. Lastly, muscle strength is associated with bone health and density.63,64 Strong muscles exert mechanical stress on bones, promoting bone remodeling and reducing the risk of fractures and falls.63,64
Our review reveals compelling evidence supporting a notable association between muscle mass and muscle strength with 10 studies stating there is a significant relationship with details of three studies stating a strong correlation, four studies with moderate correlation, and one study with low correlation; nine of these studies have a low risk of bias. While a decline in muscle strength is associated with a reduction in muscle mass, the direction of the relationship still cannot be determined as our review only included cross-sectional data. Current evidence states that a decline in muscle mass and muscle strength is not strictly parallel. Clerk and Manini 65 highlighted in their study that the decrease in muscle strength surpasses the decline in muscle mass. This discrepancy is attributed to the influence of neuromuscular factors, which are determined by the physical activity level of older adults. Current longitudinal data from the Health ABC Study show that the drop in muscle strength is significantly faster than the concurrent loss of muscle mass. Moreover, retaining or increasing muscle mass does not stop the loss of muscle strength that comes with age. Instead, they believe that the weakening of the muscles in older adults is more closely tied to problems with neural (central) activation and/or decreases in the ability of skeletal muscle to generate force on its own. 66 It is not recommended to consider muscle mass as an intermediate endpoint in interventions designed to improve functional or physical capabilities. This is because it suggests that modifications to other components of the human neuromuscular system are necessary for regulating strength. 65
We found that low muscle strength is associated with more physical performance or disability relationships than low muscle mass. Consequently, based on the scientific evidence compiled in this review, we propose that the decline in muscle strength among older individuals, without a simultaneous reduction in muscle mass, should be regarded as a distinct ailment independent of sarcopenia. This new term, dynapenia, 65 allows for early recognition of this separate condition, enabling interventions to commence sooner. Ultimately, this approach aims to prevent a decline in physical abilities that can adversely impact the quality of life for older adults.
This review has limitations with the types of data included, all of which are cross-sectional data. However, to ensure the quality of the included studies we conducted a critical review using the AXIS tool and graded the quality of evidence with GRADE. Moreover, this systematic review did not perform a meta-analysis due to the substantial heterogeneity observed among the included studies. The heterogeneity in participant characteristics, measurement methods, and statistical approaches made conducting a quantitative synthesis of the results impractical. Consequently, the review relies on a qualitative analysis of the evidence, which may limit the ability to provide precise quantitative summaries of the relationships between muscle mass, muscle strength, and physical performance in the elderly. This limitation underscores the importance of interpreting the findings cautiously and highlights the need for future research that can quantitatively synthesize these relationships to provide more robust conclusions. Despite the shortcomings in this review, this review is important, because at this time there are still few systematic review studies that examine the relationship between muscle mass and muscle strength on physical performance in older adults, so that with this review it can become new scientific evidence and become a consideration for clinicians to begin assessing low muscle strength or dynapenia as an important condition and requiring immediate intervention.
Conclusions
Muscle strength has a significant relationship to physical performance, while the relationship between muscle mass and physical performance in older adults still has inconsistent correlational evidence. As such, we acknowledge that the directionality of the relationships between muscle mass, muscle strength, and physical performance in the elderly cannot be definitively determined through this review. Therefore, future research, particularly longitudinal studies, are needed to elucidate the dynamic nature of these relationships and better understand how changes in muscle mass and strength impact physical performance over time.
Acknowledgments
We appreciate Taufik Indrajaya’s willingness to participate as the review’s third contributor.
Footnotes
Authors contribution: Conceptualization: Nur Riviati. Design and methodology: Bima Indra. Data collection: All authors. Data analysis and interpretation: All authors. Writing and drafting: Bima Indra. Critical revisions: Nur Riviati. Supervision: Taufik Indrajaya. Final approval: all authors have reviewed and approved the final version of the manuscript before submission.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: Not applicable to this review.
Consent for publication: The manuscript titled “Relationship between Muscle Mass and Muscle Strength with Physical Performance in Elderly: A Systematic Review” is hereby granted consent for publication in SAGE Open Medicine by the authors. Originality and absence of concurrent submissions are affirmed. The peer-review process and subsequent revisions are accepted, and post-publication public accessibility is acknowledged.
ORCID iD: Bima Indra  https://orcid.org/0000-0002-0745-9661
https://orcid.org/0000-0002-0745-9661
References
- 1. Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012; 11(5): 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishikawa H, Fukunishi S, Asai A, et al. Pathophysiology and mechanisms of primary sarcopenia (Review). Int J Mol Med 2021; 48: 156. [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Pickrell AM, Rossi SG, et al. Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cell pool. Hum Mol Genet 2013; 22(19): 3976–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boy EB, Indrisefani R, Fadhilah R, et al. The impact of the Covid-19 pandemic on the physical activity level of the elderly at the health center. Magna Medica Berkala Ilmiah Kedokteran dan Kesehatan 2022; 9(2): 96–101. [Google Scholar]
- 5. Hirve S, Juvekar S, Lele P, et al. Social gradients in self-reported health and well-being among adults aged 50 years and over in Pune District, India. Glob Health Action 2010; 3(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eryando T, Ariha D, Daniah, Damayanti YF, et al. Relationship of age, working and education with/regarding the quality of live of elderly. In: Proceedings of the 1st International Conference on Science, Health, Economics, Education and Technology (ICoSHEET 2019), Semarang, Indonesia, 18 December 2019. [Google Scholar]
- 7. Pereira DS, Mateo ECC De, Queiroz BZ, et al. TNF-α, IL6, and IL10 polymorphisms and the effect of physical exercise on inflammatory parameters and physical performance in elderly women. Age (Omaha) 2013; 35(6): 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and aging. J Cachexia Sarcopenia Muscle 2018; 9: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Dongen EJ, Leerlooijer JN, Steijns JM, et al. Translation of a tailored nutrition and resistance exercise intervention for elderly people to a real-life setting: adaptation process and pilot study. BMC Geriatr 2017; 17(1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azab BG, Youssif H, Maamoun M, et al. Association between physical performance and muscle strength among elderly. Egypt J Geriatr Gerontol 2015; 2(1): 33–40. [Google Scholar]
- 11. Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21(3): 300–307.e2. [DOI] [PubMed] [Google Scholar]
- 12. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48(1): 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhar M, Kapoor N, Suastika K, et al. South Asian Working Action Group on SARCOpenia (SWAG-SARCO)—a consensus document. Osteoporos Sarcopenia 2022; 8(2): 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhasin S, Travison TG, Manini TM, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc 2020; 68(7): 1410–1418. [DOI] [PubMed] [Google Scholar]
- 15. Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001; 56(5): B209–B217. [DOI] [PubMed] [Google Scholar]
- 16. Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007; 30(6): 1507–1512. [DOI] [PubMed] [Google Scholar]
- 17. Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014; 9(10): 1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reed RL, Pearlmutter L, Yochum K, et al. The relationship between muscle mass and muscle strength in the elderly. J Am Geriatr Soc 1991; 39(6): 555–561. [DOI] [PubMed] [Google Scholar]
- 19. Hayashida I, Tanimoto Y, Takahashi Y, et al. Correlation between muscle strength and muscle mass, and their association with walking speed. PLoS One 2014; 9(11): e111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferraro E, Pin F, Gorini S, et al. Improvement of skeletal muscle performance in aging by the metabolic modulator trimetazidine. J Cachexia Sarcopenia Muscle 2016; 7(4): 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verdijk LB, Snijders T, Beelen M, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc 2010; 58(11): 2069–2075. [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kementrian Kesehatan Republik Indonesia. Peraturan Menteri Kesehatan Republik Indonesia Kementerian Kesehatan Republik Indonesia. Jakarta. (2016, accessed 30 June 2023). [Google Scholar]
- 24. GRADE Working Group. GRADE welcome to the GRADE working group. From evidence to recommendations—transparent and sensible. https://www.gradeworkinggroup.org/ (2023, accessed 14 June 2023).
- 25. Jung Kim H, Hyung Kim S, Jun Park S, et al. Retrospective study influence of handgrip strength and psoas muscle index on analgesic efficacy of epidural steroid injection in patients with degenerative lumbar spinal disease. https://www.painphysicianjournal.com (2022, accessed 2 July 2023). [PubMed]
- 26. Kuan YC, Huang LK, Wang YH, et al. Balance and gait performance in older adults with early-stage cognitive impairment. Eur J Phys Rehabil Med 2021; 57(4): 560–567. [DOI] [PubMed] [Google Scholar]
- 27. Aarden JJ, Reijnierse EM, van der Schaaf M, et al. Longitudinal changes in muscle mass, muscle strength, and physical performance in acutely hospitalized older adults. J Am Med Dir Assoc 2021; 22(4): 839–845.e1. [DOI] [PubMed] [Google Scholar]
- 28. Lustosa LP, Batista PP, Pereira DS, et al. Comparison between parameters of muscle performance and inflammatory biomarkers of non-sarcopenic and sarcopenic elderly women. Clin Interv Aging 2017; 12: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alajlouni D, Bliuc D, Tran T, et al. Decline in muscle strength and performance predicts fracture risk in elderly women and men. J Clin Endocrinol Metab 2020; 105(9): dgaa414. [DOI] [PubMed] [Google Scholar]
- 30. Granic A, Hil TR, Davies K, et al. Vitamin D status, muscle strength and physical performance decline in very old adults: a prospective study. Nutrients 2017; 9(4): 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Legrand D, Vaes B, Matheï C, et al. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc 2014; 62(6): 1030–1038. [DOI] [PubMed] [Google Scholar]
- 32. Van Nieuwpoort IC, Vlot MC, Schaap LA, et al. The relationship between serum IGF-1, handgrip strength, physical performance, and falls in elderly men and women. Eur J Endocrinol 2018; 179(2): 73–84. [DOI] [PubMed] [Google Scholar]
- 33. Teraž K, Marusic U, Kalc M, et al. Sarcopenia parameters in active older adults—an eight-year longitudinal study. BMC Public Health 2023; 23(1): 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanaka K, Taoda A, Kashiwagi H. The associations between nutritional status, physical function and skeletal muscle mass of geriatric patients with colorectal cancer. Clin Nutr ESPEN 2021; 41: 318–324. [DOI] [PubMed] [Google Scholar]
- 35. Minematsu A, Hazaki K, Harano A, et al. Association between muscle strength and physical performance in Japanese elderly: the Fujiwara-kyo study. J Clin Gerontol Geriatr 2018; 9(2): 44–51. [Google Scholar]
- 36. Iwase H, Murata S, Nakano H, et al. Relationship between age-related changes in skeletal muscle mass and physical function: a cross-sectional study of an elderly Japanese population. Cureus 2022; 14: e24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nonaka K, Murata S, Shiraiwa K, et al. Effect of skeletal muscle and fat mass on muscle strength in the elderly. Healthcare (Switzerland) 2018; 6(3): 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishi NN, Tanaka N, Hirano N. Characteristics of body composition and relationship between muscle mass and muscle strength among elderly women in different age groups. Adv Aging Res 2022; 11(05): 135–149. [Google Scholar]
- 39. Irisawa H, Mizushima T. Assessment of changes in muscle mass, strength, and quality and activities of daily living in elderly stroke patients. Int J Rehabil Res 2022; 45(2): 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neves T, Fett CA, Ferriolli E, et al. Correlation between muscle mass, nutritional status and physical performance of elderly people. Osteoporos Sarcopenia 2018; 4(4): 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Almeida Silva N, Lira Júnior C, da Cunha Lisboa MG, et al. Relationship between muscle mass and neuromuscular function in the muscular strength of elderly women practicing and non-practicing physical activities. Res Soc Develop 2021; 10(17): e139101724018. [Google Scholar]
- 42. de Oliveira JCM, Gouveia CAM, Moreira LDP, et al. Correlation between muscle mass and fragility parameters in the elderly. Austin J Musculoskelet Disord 2022; 9(1): 1061–1067. [Google Scholar]
- 43. Falsarella GR, Coimbra IB, Barcelos CC, et al. Influence of muscle mass and bone mass on the mobility of elderly women: an observational study. BMC Geriatr 2014; 14(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vilaça KHC, Carneiro JAO, Ferriolli E, et al. Body composition, physical performance and muscle quality of active elderly women. Arch Gerontol Geriatr 2014; 59(1): 44–48. [DOI] [PubMed] [Google Scholar]
- 45. Kwon I, Kim JS, Shin CH, et al. Associations between skeletal muscle mass, grip strength, and physical and cognitive functions in elderly women: effect of exercise with resistive theraband. J Exerc Nutrition Biochem 2019; 23(3): 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee EJ, Lee SA, Soh Y, et al. Association between asymmetry in lower extremity lean mass and functional mobility in older adults living in the community: results from the Korean Frailty and aging cohort study. Medicine 2019; 98(45): e17882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim YH, Kim KI, Paik NJ, et al. Muscle strength: a better index of low physical performance than muscle mass in older adults. Geriatr Gerontol Int 2016; 16(5): 577–585. [DOI] [PubMed] [Google Scholar]
- 48. Duchowny KA, Peters KE, Cummings SR, et al. Association of change in muscle mass assessed by D3-creatine dilution with changes in grip strength and walking speed. J Cachexia Sarcopenia Muscle 2020; 11(1): 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chiles Shaffer N, Simonsick EM, Thorpe RJ, et al. The roles of body composition and specific strength in the relationship between race and physical performance in older adults. J Gerontol A Biol Sci Med Sci 2020; 75(4): 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elfa MM, Panghiyangani R, Widyaningtyas DA, et al. The correlation between strength of handgrip and muscle mass with elderly functional mobility at the elderly integrated service center in Pekauman Primary Health Care, Banjarmasin, Indonesia. Borneo Rev Med Sci 2020; 1(1): 1–10. [Google Scholar]
- 51. Kristiana T, Widajanti N, Satyawati R. Association between muscle mass and muscle strength with physical performance in elderly in Surabaya. Surabaya Phys Med Rehabil J 2020; 2(1): 24. [Google Scholar]
- 52. Stoever K, Heber A, Eichberg S, et al. Sarcopenia and predictors of skeletal muscle mass in elderly men with and without obesity. Gerontol Geriatr Med 2017; 3: 233372141771363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ossowski ZM, Wiech M, Ellwart M. Association between gait speed and muscle mass and strength in postmenopausal women. Balt J Health Phys Act 2019; 11(1): 106–114. [Google Scholar]
- 54. Bårdstu HB, Andersen V, Fimland MS, et al. Muscle strength is associated with physical function in community-dwelling older adults receiving home care. a cross-sectional study. Front Public Health 2022; 10: 856632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hernández-Luis R, Martín-Ponce E, Monereo-Muñoz M, et al. Prognostic value of physical function tests and muscle mass in elderly hospitalized patients. A prospective observational study. Geriatr Gerontol Int 2018; 18(1): 57–64. [DOI] [PubMed] [Google Scholar]
- 56. Palacios-Chávez M, Dejo-Seminario C, Mayta-Tristán P, et al. Physical performance and muscle strength in older patients with and without diabetes from a public hospital in Lima, Peru. Endocrinol Nutr 2016; 63: 220–229. [DOI] [PubMed] [Google Scholar]
- 57. Macedo BG, de Oliveira HSC, de Paula MV, et al. Association between inflammatory mediators, grip strength and mobility in community-dwelling elderly. Fisioterapia em Movimento 2018; 31: 1–9. [Google Scholar]
- 58. Frontera WR, Rodriguez Zayas A, Rodriguez N. Aging of human muscle: understanding sarcopenia at the single muscle cell level. Phys Med Rehabil Clin N Am 2012; 23: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fielding RA. Effects of exercise training in the elderly: impact of progressive-resistance training on skeletal muscle and whole-body protein metabolism. Proc Nutr Soc 1995; 54(3): 665–675. [DOI] [PubMed] [Google Scholar]
- 60. Koopman R. Dietary protein and exercise training in aging. Proc Nutr Soc 2011; 70: 104–113. [DOI] [PubMed] [Google Scholar]
- 61. Brotto M. Lessons from the FNIH-NIA-FDA sarcopenia consensus summit. IBMS Bonekey 2012; 9: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Visser M, Deeg DJH, Lips P, et al. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc 2000; 48(4): 381–386. [DOI] [PubMed] [Google Scholar]
- 63. Hanson ED, Srivatsan SR, Agrawal S, et al. Effects of strength training on physical function: influence of power, strength, and body composition. J Strength Cond Res 2009; 23(9): 2627–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Suchomel TJ, Nimphius S, Stone MH. The importance of muscular strength in athletic performance. Sports Med 2016; 46: 1419–49. [DOI] [PubMed] [Google Scholar]
- 65. Clark BC, Manini TM. Sarcopenia =/= Dynapenia. J Gerontol A Biol Sci Med Sci 2008; 63(8): 829–834. [DOI] [PubMed] [Google Scholar]
- 66. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 2012; 67(1): 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]