CLINICAL CONTEXT
Soft tissue sarcomas (STS) are a heterogenous group of mesenchymal malignancies with up to 60% occurring in the extremity. Radiation therapy (RT) and surgery are the mainstay of management for most large and high-grade STS in this anatomic location. While the two modalities often result in local disease control, patients frequently develop distant metastatic disease that limits median survival to less than two years.1 One strategy to treat (or prevent) metastatic disease has been to employ immune check-point inhibitors (ICI).
SU2C-SARC032 builds on the SARC028 trial, which was a multicenter, nonrandomized phase II study that identified a signal of efficacy of Pembrolizumab (an anti-PD-1 ICI) in patients with some subtypes of STS.2 The overall response rate in this study for patients with undifferentiated pleomorphic sarcoma (UPS) or dedifferentiated liposarcoma (LPS) was 23% and 10%, respectively.3
Following the results of SARC028 for patients with UPS and LPS, investigators are studying ICIs in STS in the adjuvant setting.4 Of particular interest is the combination of RT and ICI, which may have several potential synergistic benefits. First, RT results in immunogenic tumor cell death and tumor antigen release, which leads to activation of preexisting intratumoral T cells.5 Second, this release of tumor antigens may lead to recruitment of additional T cells into the TME.5 For example, a small study (n=17) of patients with UPS of the extremity and trunk showed an increased density of tumor infiltrating immune cells following RT.6
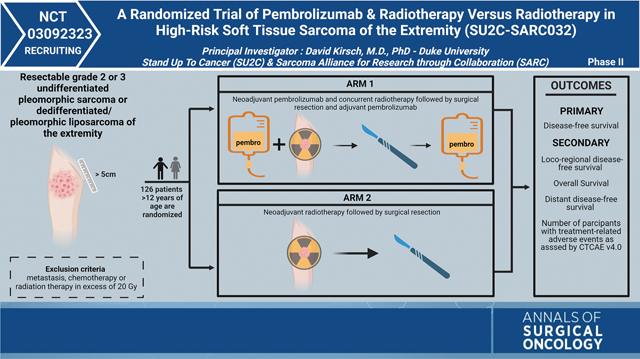
The SU2C-SARC032 trial, led by Principal Investigator Dr. David Kirsch, is a multicenter randomized phase II trial investigating the role of neoadjuvant pembrolizumab with RT and adjuvant pembrolizumab in patients with high-risk, localized UPS or dedifferentiated/pleomorphic LPS of the extremity. Patients are stratified by grade (intermediate or high grade) and randomized into two arms. Arm 1, the experimental arm, will consist of patients who receive three cycles of neoadjuvant pembrolizumab before, during, and after RT (50 Gy in 25 fractions), followed by surgical resection and adjuvant pembrolizumab for up to fourteen cycles. Pembrolizumab will be administered at 200 mg intravenously every 3 weeks. In arm 2, patients will receive neoadjuvant RT (50 Gy in 25 fractions) followed by surgical resection.
INVESTIGATOR INSIGHTS
Patients with localized, large (greater than 5 cm), intermediate to high-grade undifferentiated pleomorphic sarcoma or pleomorphic/dedifferentiated liposarcoma have a significant risk of developing metastases to the lungs after local therapy. In SARC028, a subset of patients with metastatic undifferentiated pleomorphic sarcoma or pleomorphic/dedifferentiated liposarcoma showed response to the anti-PD-1 agent pembrolizumab. Pre-clinical studies suggest that combining radiation therapy with anti-PD-1 therapy may further stimulate the anti-tumor immune response. SU2C-SARC032 was developed to test if adding pembrolizumab to preoperative radiation therapy plus adjuvant pembrolizumab activates the immune system to clear micrometastases and prevent the development of lung metastases. The primary outcome measure of the clinical trial is disease free survival at 2 years.
In addition to the important clinical question of determining whether pembrolizumab improves outcomes in this group of sarcoma patients, by analyzing the rich correlative samples from this trial, we also anticipate learning about sarcomas and their response to radiotherapy alone or combination radiotherapy and pembrolizumab. Pre-treatment biopsies and resection samples will be compared by RNA sequencing to define changes in the immune cell composition of tumors. Peripheral blood mononuclear cells and plasma before, during, and after neoadjuvant therapy and after surgery will be analyzed for changes in the peripheral immune response and in circulating tumor DNA (ctDNA), respectively. If the results of SU2C-SARC032 trial show that the combination of pembrolizumab with preoperative radiation therapy plus adjuvant pembrolizumab improves outcomes for high-risk, localized UPS or pleomorphic/dedifferentiated LPS, then pembrolizumab may become a viable option for these patients in the future.
METHODS
Supplementary Material
ACKNOWLEDGMENTS
The SU2C-SARC032 trial is made possible by collaborative efforts between the Stand Up to Cancer (SU2C) foundation and the Sarcoma Alliance for Research through Collaboration (SARC), a non-profit cancer research organization. SARC and the Merck Sharp & Dohme LLC groups are the primary study sponsors for this trial. Conflict of interest statements by engaged authors have been provided.
Footnotes
DISCLOSURES DGK has received research funding from XRAD Therapeutics, Bristol Myers Squibb, Varian Medical Systems, Merck Sharp and Dohme LLC. DGK owns stock and is on the scientific advisory board of Lumicell, Inc., which is commercializing intraoperative imaging technology. DGK is a cofounder of XRAD Therapeutics, which is developing radiosensitizers. The other co-authors report no conflicts of interest.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1245/s10434-022-12762-z.
REFERENCES
- 1.Mowery YM, Ballman KV, Riedel RF, et al. SU2C-SARC032: A phase II randomized controlled trial of neoadjuvant pembrolizumab with radiotherapy and adjuvant pembrolizumab for high-risk soft tissue sarcoma. Journal of Clinical Oncology. 2018;36(15):588. 10.1200/JCO.2018.36.15_suppl.TPS11588. [DOI] [Google Scholar]
- 2.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in-advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. The Lancet Oncology. 2017;18(11):1493–501. 10.1016/s1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess MA, Bolejack V, Schuetze S, et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. Journal of Clinical Oncology. 2019;37(15):11015–11015. 10.1200/JCO.2019.37.15_suppl.11015. [DOI] [Google Scholar]
- 4.Italiano A, Bellera C, D’Angelo S. PD1/PD-L1 targeting in-advanced soft-tissue sarcomas: a pooled analysis of phase II trials. Journal of Hematology & Oncology. 2020;13(1):55. 10.1186/s13045-020-00891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldi GG, Gronchi A, Tazzari M, Stacchiotti S. Immunotherapy in soft tissue sarcoma: current evidence and future perspectives in a variegated family of different tumor. Expert Review of Anticancer Therapy. 2022;2:1–13. 10.1080/14737140.2022.2065986. [DOI] [PubMed] [Google Scholar]
- 6.Keung EZ, Tsai JW, Ali AM, et al. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: Rationale for combination neoadjuvant immune checkpoint inhibition and radiotherapy. Oncoimmunology. 2018;7(2):e1385689. 10.1080/2162402x.2017.1385689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


