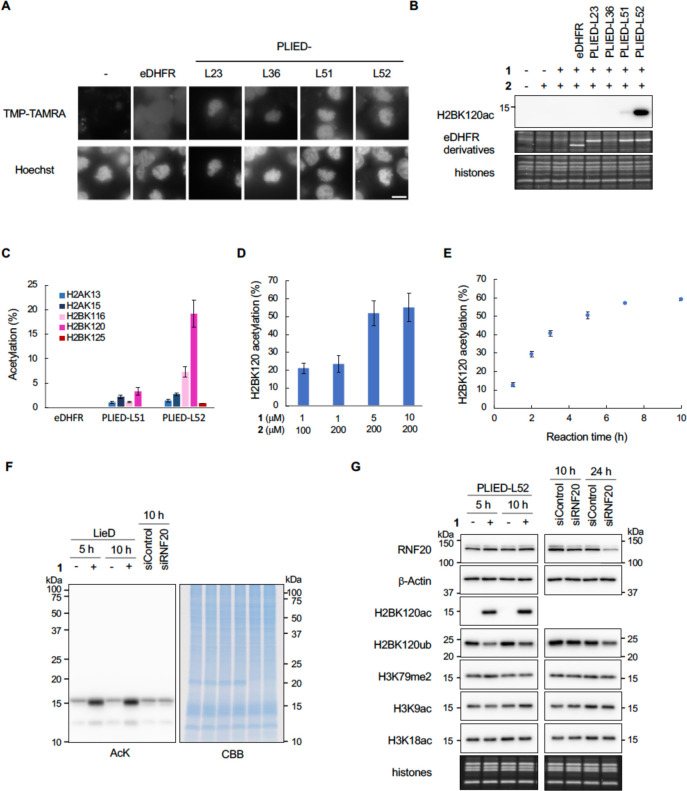
Figure 4.
In-cell histone acetylation reaction by PLIED/1. A, Subcellular localization of PLIED. eDHFR-FLAG- or PLIED-FLAG-transfected HEK293T cells were treated with nocodazole (330 nM) for 4 h, followed by TMP-TAMRA (10 μM, chemical structure is shown in Figure S3A) with nocodazole for 1 h. DNA was stained with Hoechst 33342 to visualize chromatin distribution. Representative images of mitotic cells are shown. Scale bar, 10 μm. B, In-cell histone acetylation by PLIED/1 systems. eDHFR-FLAG- or PLIED-FLAG-transfected HEK293T cells were incubated with or without acetyl donor 2 (100 μM) and TMP-BAHA 1 (1 μM) at 37 °C for 5 h. Whole-cell extracts were immunoblotted with anti-H2BK120ac antibody. The expressed eDHFR derivatives and histones were visualized by Oriole staining. Representative data of two independent experiments are shown. C, Acetylation yields of indicated lysine residues on histones from PLIED/1-treated HEK293T cells as in B. The yield was determined by LC–MS/MS. D, Optimization of histone acetylation by PLIED-L52. PLIED-L52-FLAG-transfected HEK293T cells were incubated with TMP-BAHA 1 and acetyl donor 2 at the indicated concentrations at 37 °C for 5 h. E, Time course analysis of histone acetylation by PLIED-L52/1. PLIED-L52-FLAG-transfected HEK293T cells were incubated with TMP-BAHA 1 (5 μM) and acetyl donor 2 (200 μM) at 37 °C for the indicated time. Acetylation yields were analyzed by LC–MS/MS. For LC–MS/MS data, error bars represent the range of two independent experiments (C–E). F, Histone-selectivity of PLIED-L52/1-mediated acetylation. PLIED-L52-FLAG-transfected HEK293T cells were incubated with or without TMP-BAHA 1 (5 μM) in the presence of acetyl donor 2 (200 μM) at 37 °C for 5 or 10 h. For knockdown of RNF20, HEK293T cells were transfected with control or RNF20 siRNA and harvested after 10 h. The lysine acetylation levels of proteins in the whole-cell extract were detected by immunoblotting using anti-AcK antibody. Proteins were visualized by CBB staining. Representative data of two independent experiments are shown. G, Immunoblotting analysis of the PLIED-L52/1-mediated in-cell acetylation. HEK293T cells were treated as in F. To assess levels of RNF20, whole-cell extracts were immunoblotted with anti-RNF20. β-Actin was used as a loading control. To assess levels of histone PTMs, histones were acid-extracted from the cells and analyzed by immunoblotting with the indicated antibodies. The loading amounts of histones were visualized with Oriole staining. Representative data of two independent experiments are shown.