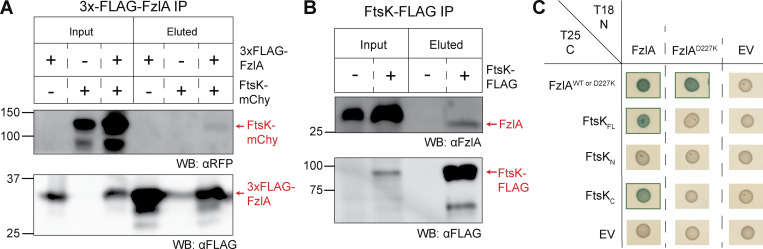
Figure 7.
FzlA interacts with FtsKC via the FzlA C-terminal tail. (A and B) Western blots of whole cell lysates or eluates from an immunoprecipitation (IP) using α-FLAG resin. Ladder values to the left of the blots are in kDa. Red arrows to the right of the blots indicate to what protein the band is attributed. The presence or absence of the fusion proteins in the strains used are indicated above the blots by plus (+) or minus (−) signs, respectively. (A) 3xFLAG-FzlA immunoprecipitation. Blots were incubated with primary antibodies recognizing either mCherry (αRFP) or FLAG (αFLAG). Lane 1 and 4, fzlA::3xFLAG-fzlA (EG2217). Lane 2 and 5, ftsK:: ftsK-mChy (EG2427). Lane 3 and 6, fzlA::3xFLAG-fzlA; ftsK:: ftsK-mChy (EG2428). (B) FtsK-FLAG immunoprecipitation. Blots were incubated with primary antibodies recognizing either FzlA (αFzlA) or FLAG (αFLAG). Lane 1 and 3, WT (EG865). Lane 2 and 4, ftsK::ftsK-FLAG (EG742). (C) BTH results for interaction between FzlA variants and full-length FtsK or its domains. The adenylyl cyclase subunits T18 (left) and T25 (top) are fused to proteins via the N-terminus or C-terminus, respectively. A green box around the representative spot image indicates that the three biological triplicates were positive for induction of the cAMP-dependent β-galactosidase reporter, indicating a positive interaction. Some data in C is duplicated in Fig. S5 A. Source data are available for this figure: SourceData F7.