FIGURE 3.
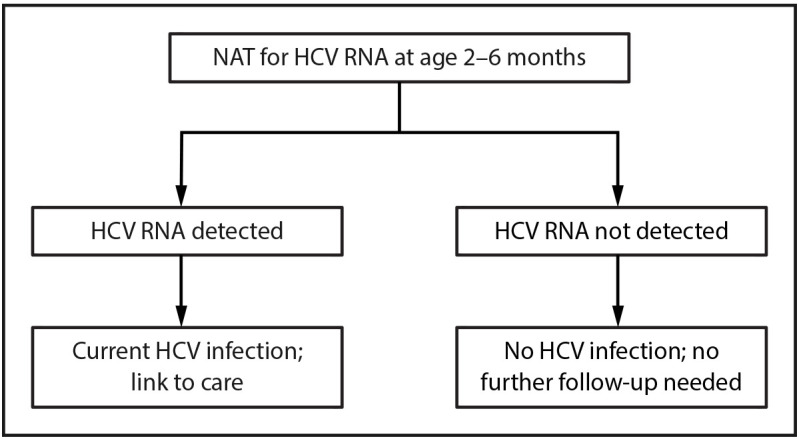
Algorithm for hepatitis C virus testing of perinatally exposed children — United States, 2023*,†,§,¶
Abbreviations: FDA = Food and Drug Administration; HCV = hepatitis C virus; NAT = nucleic acid test.
* Perinatally exposed children are children born to pregnant persons with HCV infection.
† Perinatally exposed children aged 7–17 months who have not previously been tested also should receive a NAT for HCV RNA.
§ Off-label use of an FDA-approved diagnostic test requires validation by the testing laboratory.
¶ No further follow-up needed after a negative HCV RNA performed at age 2–6 months unless clinically warranted (i.e., clinical symptoms or signs or laboratory findings consistent with hepatitis C).
