Abstract
The physiological impact of cannabinoid receptor agonists is of great public health interest due to their increased use in recreational and therapeutic contexts. However, the body of literature on cannabinoid receptor agonists includes multiple confounding variables that complicate comparisons across studies, including route of administration, timeline across which phenotypes are observed, agonist dose, and sex of the study cohort. In this study, we characterized the impact of sex and route of administration on Δ9-tetrahydrocannabinol (THC)-induced changes in cardiopulmonary phenotypes in mice. Using noninvasive plethysmography and telemetry, we monitored heart rate and respiration in the same cohort of animals across aerosol, oral gavage, subcutaneous, and intraperitoneal administrations of THC (0-30 mg/kg THC for oral gavage, subcutaneous, and intraperitoneal, and 0-300 mg/ml THC for aerosol). All routes of THC administration altered respiratory minute volume and heart rate, with the direction of effects typically being consistent across dependent measures. THC primarily decreased respiration and heart rate, but females given oral gavage THC showed increased heart rate. Intraperitoneal and subcutaneous THC produced the longest-lasting effects, including THC-induced alterations in physiological parameters for up to 10 h, whereas effects of aerosolized THC were short lived. The fastest onset of effects of THC occurred for aerosolized and intraperitoneal THC. Altogether, the work herein establishes the impact of dosing route on THC-induced heart rate and respiratory alteration in male and female mice. This study highlights important differences in the timeline of cardiopulmonary response to THC following the most common preclinical routes of administration.
Keywords: cardiovascular effects, delta-9-tetrahydrocannabinol, plethysmography, route of administration, sex differences, telemetry
1.0. Introduction
With the number of states in the U.S. decriminalizing or legalizing cannabis use rising, the prevalence of adult use of cannabis for medicinal and recreational purposes has increased [1]. Concomitantly, augmented concentrations of Δ9-tetrahydrocannabinol (THC), the primary psychoactive substituent of the cannabis plant, have become common with targeted breeding of the plant for high THC concentrations [2, 3]. THC-containing products include edibles, e-liquids and other concentrate formulations (e.g., wax, shatter) that may contain over 60% THC, as compared to an average of about 5% THC from 1993-2003 [3-5]. In addition to being smoked like a traditional marijuana cigarette, these products may be ingested orally or vaped. The degree to which route of administration affects the subsequent pharmacological in vivo potency, efficacy and time course of THC-containing products has received limited research attention in humans [6-15] or in animal models that have been used to investigate mechanisms underlying cannabinoid actions [16-23].
Oral administration is the most employed route of dosing in clinical trials using cannabis and cannabinoids [6], though recent studies have tested for differences among routes of administration. THC is lipophilic, leading to poor oral bioavailability and limited uptake through transdermal administration [24]. In humans, orally-administered cannabis has delayed detection and a lower peak concentration of THC in blood relative to inhaled administration [24-26], though detected analytes in urine are higher among participants receiving cannabis through oral administration compared to vaporized cannabis [10]. Sex differences in cannabis metabolism add further complexity to our understanding of pharmacokinetics across routes of administration [27]. Furthermore, the vehicle used for compound formulation may also impact the onset and duration of cannabinoid-mediated effects, and the ideal route of administration and tissue distribution may differ among cannabinoids [21].
Examples from the literature suggest that route of cannabinoid administration has an impact on both clinically relevant disease phenotypes and the side effects of increased cannabinoid signaling. In a drug discrimination study using rats, routes of THC administration with first-pass metabolism had similar potencies, inhaled THC had a swifter onset but shorter duration of action, and subcutaneous (s.c.) dosing produced lower potency but longer duration of action [16]. Among rats, oral administration leads to higher levels of cannabinoids in the brain and a higher degree of behavioral effects relative to inhaled and s.c. administration [17]. In a study of intraocular pressure using rabbits, topical and intravenous administration of a cannabinoid each reduced the phenotype of interest, but topical administration had no significant impacts on behavioral and cardiovascular phenotypes [28]. A meta-analysis of human trials suggests that compound form and route of administration may impact the antinociceptive effects of THC across clinically-relevant pain pathologies [12]. Among a cohort of participants suffering endometriosis, cannabis use was associated with improvements in pain, mood, and gastrointestinal symptoms, with inhalation leading to greater improvements in pain and oral administration leading to greater improvements in mood and gastrointestinal symptoms [15].
To date, most preclinical research has focused on effects of intraperitoneal (i.p.) injection as a route of administration in rodent models of cannabinoid effects. Further, the most intense interest has resided in examining centrally mediated, abuse-related effects of THC, including its acute reinforcing and rewarding effects [29-33], discriminative stimulus [34, 35] effects, and the development of tolerance and dependence after repeated administration [36-39]. In contrast, the effects of THC on peripheral systems have not been as well delineated, partly due to delays in miniaturization and adaptation of equipment needed to measure functioning of peripheral systems in freely moving rodents, the animals most commonly used in preclinical biomedical research. Development of whole-body plethysmography and cardiovascular telemetry systems, and their recent simultaneous use [40], has yielded procedures that may provide a more nuanced examination of drugs on peripheral functioning across time. In the present study, we investigated effects of THC on respiratory and cardiovascular functioning in mice across intraperitoneal (i.p.) and s.c. injection, oral gavage (p.o.), and exposure to aerosolized THC. The goal was to determine the relative potency, efficacy, and time course across route of administration in each sex. The hypothesis was that THC would lower respiratory and cardiovascular functioning, and that different routes of administration would have different time courses of effects.
2.0. Materials and methods
2.1. Subjects
Adult male and female C57BL/6 mice (29-30 g for males and 25-26 g for females at the beginning of the experiment; Envigo, Frederick, MD) were surgically implanted with HD-X10 telemetry probes at DSI [Data Sciences International (DSI), Inc., St. Paul, MN] and allowed to recover before shipment to RTI. Upon arrival, mice were individually housed in polycarbonate cages with hardwood bedding in a temperature-controlled (20–22°C) environment with a 12 h light-dark cycle (lights on at 7 am). Mice were fed approximately 3 g of food per day and water was available ad libitum in their home cages. All studies were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals [41], complied with the ARRIVE guidelines, and were approved by our Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
2.2. Apparatus
Mouse respiration was assessed in a Buxco FinePointe Whole Body Plethysmography System (DSI). During measurement, mice were confined to cylindrical Plexiglas chambers (7.5 cm diameter X 8 cm height) located in a laboratory with 12-h light-dark cycle (lights on at 7 am). Each plethysmography chamber rested on a RPC-1 Physiotel telemetry receiver platform (DSI), with associated Matrix 2.0 transducer (DSI) interface with the data acquisition computer, to allow simultaneous measurement of respiratory and cardiac parameters. Measurement of cardiac functioning was accomplished by means of surgically implanted HD-X10 mouse telemetry probes. Respiratory and cardiac data collection was controlled by FinePointe (version 2.7.0.11788; DSI) and Ponemah (version 6.50; DSI) software, respectively.
For the aerosol route of administration, THC aerosol was delivered to mouse-sized chambers (10 cm X 10 cm X 10 cm; EZ-177 Sure-Seal, E-Z-Anesthesia, Palmer, PA) via a commercially available vaporizer (Model SVS-200, Scientific Vapor, Bend, OR) connected to an e-vape controller (LJARI, La Jolla, CA), as described previously [42]. Airflow was constant (1 L/min) and aerosol was dispensed from an e-cigarette tank (Innokin Zenith, Element Vape, South El Monte, CA) via Tygon tubing (Fisher Scientific, Pittsburgh, PA, USA). The system was configured at 10 W using a 1.6 Ω atomizer (Innokin Z-Coil 1.6 Ω, Element Vape, South El Monte, CA).
2.3. Chemicals
Δ9-Tetrahydrocannabinol (National Institute on Drug Abuse, NIDA, Rockville, MD) was suspended in a 7.8% polysorbate 80 (Fisher Scientific) and 92.2% saline (Patterson Vet Supply, Blythewood, SC) mixture for systemic administration. Intraperitoneal (i.p.), subcutaneous (s.c.), and oral gavage (p.o.) injections of THC or vehicle were given at a volume of 10 ml/kg. For aerosolization, THC was mixed in propylene glycol (PG) (Fisher Scientific). Concentrations for aerosol administration are expressed as mg/ml in the e-cigarette tank and may not be representative of the actual amount of drug administered to the mouse.
2.4. Procedure
The overall study design involved repeated sessions of simultaneous and automated measurement of respiratory and cardiac parameters in unrestrained mice after administration of THC via four routes of administration: i.p., oral, s.c., and aerosol exposure. Habituation to the chambers was not conducted prior to the beginning of the experiments and was not incorporated into the experimental design. Mice (n=4/sex) were first evaluated with vehicle and all doses of i.p. THC, and then with vehicle and all doses of p.o. THC, with each dose-effect curve conducted in ascending sequence. Subsequently, one mouse of each sex was sacrificed due to declining health. The remaining mice (n=3/sex) were tested next with aerosolized THC, before beginning tests with s.c. THC. Again, doses/concentrations were administered in ascending order for each of these latter routes of administration. During each 18-h session, measures of respiratory and cardiac function were recorded in 1-min bins. No food or water was available during the experimental session.
For the i.p., p.o., and s.c. routes of administration, mice were administered THC or vehicle and placed immediately into individual plethysmography chambers. Subsequently, respiration and cardiac functioning were measured for 18 h before mice were removed from the chambers and returned to their home cages where they had access to water. Mice were fed immediately after removal from the chambers. For aerosol administration, exposures occurred in the aerosol chambers prior to placement in the plethysmography chambers. Mice were exposed to each THC concentration for ten 6-s infusions with 12-s inter-infusion intervals over a 180-s exposure session. For all routes of administration, test sessions were initiated during the afternoon between 3-4 pm and occurred no more than once weekly for each mouse.
2.5. Data analysis
Minute volume, the amount of air intake per min, is a function of tidal volume (amount of air intake per breath) and frequency (breaths/min) and represents a measure of overall respiratory functioning. We have included figures showing side-by-side comparison of minute volume, frequency, and tidal volume in the supplement (Figures S1-S8). Minute volume (ml/min) and heart rate (beats/min) were chosen as the primary respiratory and cardiac variables of interest for this study. For the purposes of analysis, data for the first 60 min were collated into 5-min bins and data collected from 90-600 min post-administration were collated into 30-min bins. The first 60 min period covers acclimation and onset of drug effect. Data for each time period within each route of administration were initially analyzed using separate three-factor split-plot analysis of variance (ANOVA), with sex as the between subject variable and dose and time as the within subject variables. Results of all of these ANOVAs showed that the main effects for sex were not statistically significant for either variable (heart rate or minute volume). Consequently, the data were re-analyzed using separate two-factor (dose X time) repeated measures ANOVA separately for each sex. Further, despite lack of statistically significant main effects for sex, the data were analyzed and presented in a manner that allowed visualization of the effects of dose and time in each sex (versus combining data across sex). Significant ANOVAs were followed by Tukey post hoc tests (α = 0.05) to determine differences between means. Number Cruncher Statistical Systems software (NCSS Statistical Software, Kaysville, UT, USA) was used for all analyses, and figures were created with GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA).
3.0. Results
Across routes of administration, vehicle-treated mice showed higher minute volume (MV) and heart rate following vehicle administration and introduction to plethysmography chambers compared to later in the session (Figures 1-4). This is most evident in the animals’ first exposure to the plethysmography chambers (Figure 1), and these initial elevations decreased across the first hour of data collection (left panels).
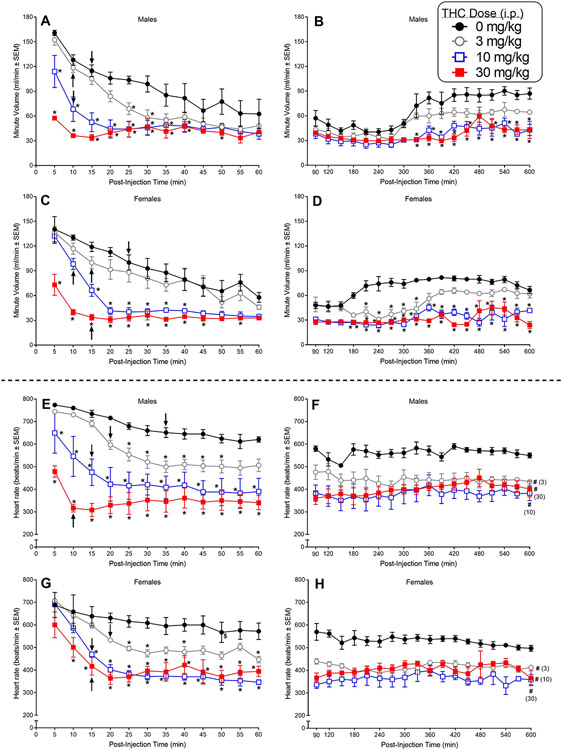
Fig. 1.
The top four panels (A-D) show the effects of intraperitoneal (i.p.) THC on respiratory minute volume in adult male and female C57BL/6 mice (n=4/sex). The effects of i.p. THC on minute volume are shown in 5-min bins over the first 60 min after injection (panel A and C in males and females, respectively) and subsequently, in 30-min bins, from 90-600 min following injection (panels B and D in males and females, respectively). The bottom four panels (E-H) show the effects of i.p. THC on heart rate in the same mice. The effects of i.p. THC on heart rate are shown in 5-min bins over the first 60 min after injection (panel E and G in males and females, respectively) and subsequently, in 30-min bins, from 90-600 min following injection (panels F and H in males and females, respectively). Asterisks (*) indicate a significant difference between vehicle and the indicated dose at a given time (dose X time interaction) (p<0.05). Pound signs (#) indicate a significant main effect of dose (compared to vehicle) across time (p<0.05). Significant differences between the initial measurement at 5-min and measurements at a single specific time point or for the remaining time points at the specific dose are indicated by a dollar sign ($) or an arrow, respectively (p<0.05).
3.1. Intraperitoneal administration
Figure 1 shows the effects of different doses of i.p. THC on MV (panels A-D) and heart rate (panels E-H) in mice of both sexes expressed in 5-min bins for the first 60 min after injection (left panels) and in 30-min bins from 90 to 600 min post-injection (right panels).
3.1.1. Plethysmography (i.p.).
In males, 10 and 30 mg/kg i.p. THC significantly suppressed MV compared to vehicle from 5 to 40 min post-injection [Fig. 1A; dose X time interaction: F(33,99)=7.19, p<0.0001]. The 30 mg/kg dose also suppressed MV at the 50-min time point. In addition, 3 mg/kg i.p. THC significantly attenuated MV at the 25- and 30-min time points, an effect that was not observed in females (see below). Vehicle-treated male mice showed significant habituation to the plethysmography chambers as the session progressed (Fig. 1A), with MV at its peak when the session started and the initial decrease occurring at 15 min post-injection (compared to the 5-min time point). Significant decreases from the initial peak MV occurred at the 10-min time point after administration of 3 or 10 mg/kg i.p. THC whereas 30 mg/kg THC produced substantial suppression of MV at 5 min without further significant decreases over time. Over the course of 90-600 min post-injection, the MV of vehicle-treated male mice remained steady until 270 min and then increased to a level plateau starting at 360 min (Fig. 1B). Treatment with 10 or 30 mg/kg THC, but not 3 mg/kg, significantly attenuated respiratory MV at many of the later time points [dose X time interaction: F(51,153)=2.71, p<0.0001].
In females, the pattern of changes in MV over time resembled that seen in males. Doses of 10 and 30 mg/kg i.p. THC, but not 3 mg/kg, significantly suppressed MV compared to vehicle from 5 min (30 mg/kg) or 15 min (10 mg/kg) to 40 min post-injection [Fig. 1C; dose X time interaction: F(33,99)=4.50, p<0.0001]. The 30 mg/kg dose continued to suppress MV at the 45- and 55-min time points. Vehicle-treated female mice showed significant habituation to the plethysmography chambers as the session progressed (Fig. 1C), with MV at its peak when the session started and the initial decrease occurring at 25 min post-injection (compared to the 5-min time point). Significant decreases from the initial peak MV occurred sooner after administration of THC (time of initial decrease compared to respective 5-min time point ranged from 10-15 min), reflecting a combination of habituation (as occurred with vehicle) and onset of drug effect. Over the course of 90-600 min post-injection, the MV of vehicle-treated female mice increased to reach a steady plateau starting at 210 min (Fig. 1D). In contrast, when the females were treated with any THC dose, this increase in MV was delayed (3 mg/kg) or did not occur (10 and 30 mg/kg), resulting in significant attenuation of respiration (compared to vehicle) at some or most time points across the 600 min post-injection time span [Fig. 1D; dose X time interaction: F(51,153)=3.67, p<0.0001].
3.1.2. Telemetry (i.p.).
Concomitant with its respiratory depressant effects, i.p. THC dose-dependently decreased heart rate in mice of both sexes. While vehicle-treated males exhibited only minor decline in heart rate over the entire 600 min period, males injected i.p. with THC showed pronounced significant time- and dose-dependent decreases in heart rate, with all 3 doses of i.p. THC producing sustained decreases in heart rate (as compared with vehicle) beginning at 5 min post-injection for 10 and 30 mg/kg THC and at 25 min after injection for the 3 mg/kg dose [Fig. 1E; dose X time interaction: F(33,99)=4.22, p<0.000001] and continuing throughout the 600 min period [Fig. 1F; dose main effect: F(3,9)=17.91, p<0.0004].
Similarly, THC also produced significant dose- and time-dependent decreases in heart rate in females, with 10 and 30 mg/kg producing suppressed heart rate at 15 and 10 min post-injection, respectively, and 3 mg/kg decreasing heart rate by 25 min after injection (each dose as compared with vehicle) [Fig. 1G; dose X time interaction: F(33,99)=3.12, p=0.000007]. THC-induced decreases endured throughout the 600 min period for all doses [Fig. 1H; dose main effect: F(3,9)=16.05, p<0.0006].
3.2. Oral administration
Figure 2 shows the effects of different doses of p.o. THC on MV (panels A-D) and heart rate (panels E-H) in mice of both sexes expressed in 5-min bins for the first 60 min after injection (left panels) and in 30-min bins from 90 to 600 min post-injection (right panels).
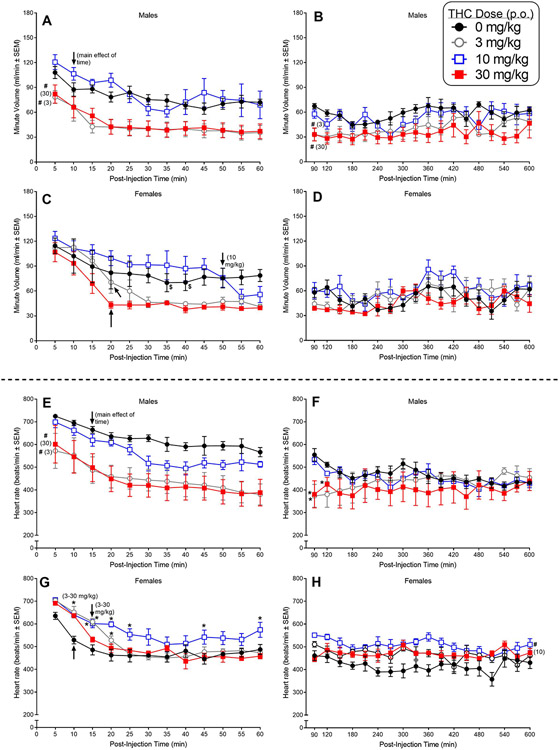
Fig. 2.
The top four panels (A-D) show the effects of oral (p.o.) THC on respiratory minute volume in adult male and female C57BL/6 mice (n=4/sex). The effects of p.o. THC on minute volume are shown in 5-min bins over the first 60 min after injection (panel A and C in males and females, respectively) and subsequently, in 30-min bins, from 90-600 min following injection (panels B and D in males and females, respectively). The bottom four panels (E-H) show the effects of p.o. THC on heart rate in the same mice. The effects of p.o. THC on heart rate are shown in 5-min bins over the first 60 min after injection (panel E and G in males and females, respectively) and subsequently, in 30-min bins, from 90-600 min following injection (panels F and H in males and females, respectively). Asterisks (*) indicate a significant difference between vehicle and the indicated dose at a given time (dose X time interaction) (p<0.05). Pound signs (#) indicate a significant main effect of dose (compared to vehicle) across time (p<0.05). Significant differences between the initial measurement at 5-min and measurements at a single specific time point or for the remaining time points at the specific dose are indicated by a dollar sign ($) or an arrow, respectively (p<0.05).
3.2.1. Plethysmography (p.o.).
In male mice, 3 and 30 mg/kg p.o. THC significantly suppressed MV (compared to vehicle) for the first 60 min after injection [Fig. 2A; main effect of dose for 60 min: F(3,9)=30.39, p=0.00005] and, to a lesser extent, over the remainder of the 600-min session [Fig. 2B; main effect of dose 90-600 min: F(3,9)=10.92, p=0.002]. MV also decreased across time during the first 60 min (compared to the 5-min timepoint), starting at 10 min post-injection [Fig. 2A; main effect of time: F(17,51)=3.18, p=0.0007].
Visual inspection of the gavage graph for females showed that the 3 and 30 mg/kg p.o. doses of THC, but not 10 mg/kg, appeared to decrease MV (Fig. 2C); however, post-hoc analysis of the ANOVA on these data revealed that neither of these two doses significantly decreased MV as compared to vehicle, although both doses significantly suppressed MV over time (compared to the 5-min timepoint) beginning at 20 min post-injection [Fig. 2D; dose X time interaction: F(33,99)=2.21, p=0.001]. Over the ensuing time to 600 min post-injection, THC also did not significantly affect MV (Fig. 2D). Power estimates suggest that the p.o. experiment in females may have been underpowered for the dose and dose X time effects in these ANOVAs (estimated power range with Greenhouse-Geisser correction = 0.20-0.46).
3.2.2. Telemetry (p.o.).
In males, 3 and 30 mg/kg THC, but not 10 mg/kg, significantly decreased heart rate compared to vehicle during the first 60 min post-gavage [Fig. 2E; main effect of dose: F(3,9)=8.66, p=0.005]. While these decreases also were observed during the initial bins of the 90-600 min period [dose X time interaction: F(51,153)=2.01, p=0.0006], they were transient and had disappeared by 180 min post-injection [Fig. 2F].
The pattern of THC’s effects on heart rate in females was opposite that observed in males. In females, p.o. administration of THC increased heart rate compared to vehicle at several times over the first 60 min post-injection [Fig. 2G; dose X time interaction: F(33,99)=3.33, p=0.000002]. These increases occurred primarily during the initial 20 min of the session at the 3 and 10 mg/kg doses and dissipated over time. At 90-min post-gavage, heart rates after administration of 3 or 30 mg/kg p.o. THC did not differ from those after vehicle, while heart rates continued to be increased after 10 mg/kg p.o. THC in female mice [Fig. 2H; main effect of dose: F(3,9)=6.60, p=0.01].
3.3. Subcutaneous administration
Figure 3 shows the effects of different doses of s.c. THC on MV (panels A-D) and heart rate (panels E-H) in mice of both sexes expressed in 5-min bins for the first 60 min after injection (left panels) and in 30-min bins from 90 to 600 min post-injection (right panels).
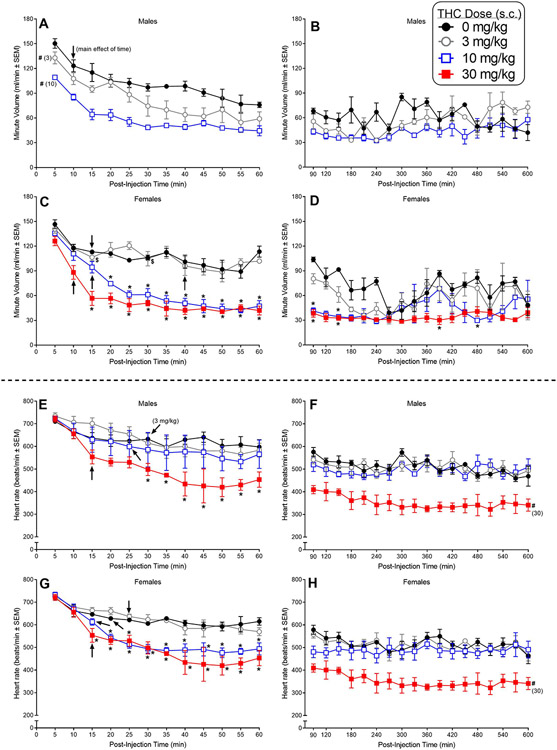
Fig. 3.
The top four panels (A-D) show the effects of subcutaneous (s.c.) THC on respiratory minute volume in adult male and female C57BL/6 mice (n=3/sex). The effects of s.c. THC on minute volume are shown in 5-min bins over the first 60 min after injection (panel A and C in males and females, respectively) and subsequently, in 30-min bins, from 90-600 min following injection (panels B and D in males and females, respectively). The bottom four panels (E-H) show the effects of s.c. THC on heart rate in the same mice. The effects of s.c. THC on heart rate are shown in 5-min bins over the first 60 min after injection (panel E and G in males and females, respectively) and subsequently, in 30-min bins, from 90-600 min following injection (panels F and H in males and females, respectively). Asterisks (*) indicate a significant difference between vehicle and the indicated dose at a given time (dose X time interaction) (p<0.05). Pound signs (#) indicate a significant main effect of dose (compared to vehicle) across time (p<0.05). Significant differences between the initial measurement at 5-min and measurements at a single specific time point or for the remaining time points at the specific dose are indicated by a dollar sign ($) or an arrow, respectively (p<0.05).
3.3.1. Plethysmography (s.c.).
In males, MV data for 30 mg/kg s.c. THC were lost due to equipment malfunction; however, both of the other s.c. THC doses (3 and 10 mg/kg) significantly decreased MV over the first 60 min of the plethysmography session compared to vehicle [Fig. 3A; main effect of dose: F(2,4)=43.69, p=0.002]. Habituation over the first 60 min was also observed, with MV values at 10 min and later significantly decreased compared to MV at the 5-min time point [Fig. 3A; main effect of time: F(11,22)=95.60, p<0.000001]. Over the course of 90-600 min post-injection, neither dose of THC produced significant decreases in MV compared to vehicle [Fig. 3B].
In females, 10 and 30 mg/kg s.c. THC, but not 3 mg/kg, significantly suppressed MV compared to vehicle from 15 min (30 mg/kg) or 20 min (10 mg/kg) to 60 min post-injection [Fig. 3C; dose X time interaction: F(33,66)=4.28, p<0.000001]. Significant decreases compared to the 5-min timepoint occurred for vehicle and all three THC doses [Fig. 3C; dose X time interaction: F(33,66)=4.28, p<0.000001]. The 30 mg/kg dose, but not the 3 or 10 mg/kg doses, produced prolonged suppression of MV, enduring throughout the 600 min time period [Fig. 3D; main effect of dose: F(3,6)=19.34, p=0.002].
3.3.2. Telemetry (s.c.).
Concomitant with its respiratory depressant effects, s.c. THC dose-dependently decreased heart rate in male mice compared to vehicle, albeit it did so only at the 30 mg/kg dose. THC (30 mg/kg, s.c.) significantly decreased heart rate compared to vehicle starting at 30 min post-injection [Fig. 3E; dose X time interaction: F(33,66)=2.59, p=0.01] and continuing for the remaining time [Fig. 3F; main effect of dose: F(3,6)=26.98, p=0.0007]. During the first 60 min of the session, decreases in heart rate over time compared to the first 5-min time were also observed in mice that received any of the THC doses [Fig. 3E; dose X time interaction: F(33,66)=2.59, p=0.01].
S.c. THC also decreased heart rate in female mice. THC (10 and 30 mg/kg, s.c.) significantly decreased heart rate compared to vehicle starting at 15-20 min post-injection [Fig. 3G; dose X time interaction: F(33,66)=7.44, p<0.000001]. Further, 30 mg/kg continued to suppress heart rate for the remaining time up to 600 min post-injection [Fig. 3H; main effect of dose: F(3,6)=11.51, p=0.007]. During the first 60 min of the session, significant habituation (compared to 5-min time) was also observed in mice that received vehicle and those that received any of the THC doses, starting at 15-25 min post-injection [Fig. 3G; dose X time interaction: F(33,66)=7.44, p<0.000001].
3.4. Aerosol exposure
Figure 4 shows the effects of different concentrations of aerosolized THC on MV (panels A-D) and heart rate (panels E-H) in mice of both sexes expressed in 5-min bins for the first 60 min after exposure (left panels) and in 30-min bins from 90 to 600 min post- exposure (right panels).
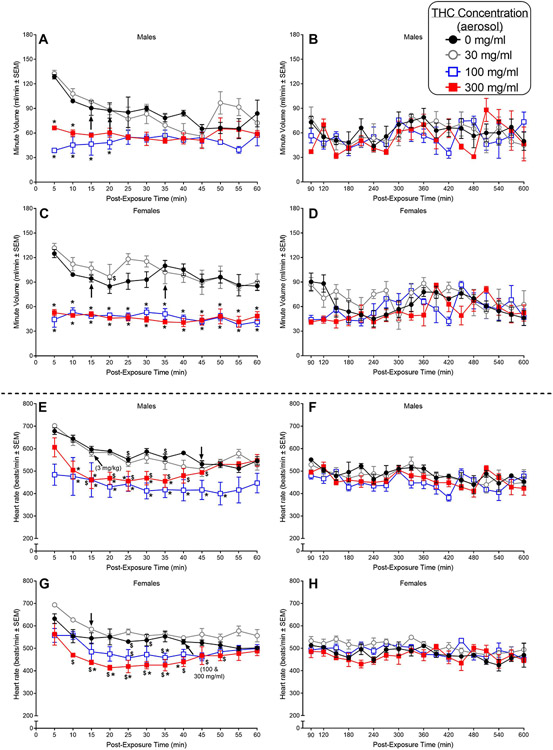
Fig. 4.
The top four panels (A-D) show the effects of THC administered via aerosol exposure on respiratory minute volume in adult male and female C57BL/6 mice (n=3/sex). The effects of aerosolized THC on minute volume are shown in 5-min bins over the first 60 min after injection (panel A and C in males and females, respectively) and subsequently, in 30-min bins, from 90-600 min following injection (panels B and D in males and females, respectively). The bottom four panels (E-H) show the effects of aerosolized THC on heart rate in the same mice. The effects of aerosolized THC on heart rate are shown in 5-min bins over the first 60 min after injection (panel E and G in males and females, respectively) and subsequently, in 30-min bins, from 90-600 min following injection (panels F and H in males and females, respectively). Asterisks (*) indicate a significant difference between vehicle and the indicated dose at a given time (dose X time interaction) (p<0.05). Pound signs (#) indicate a significant main effect of dose (compared to vehicle) across time (p<0.05). Significant differences between the initial measurement at 5-min and measurements at a single specific time point or for the remaining time points at the specific dose are indicated by a dollar sign ($) or an arrow, respectively (p<0.05).
3.4.1. Plethysmography (aerosol).
In males, both 100 and 300 mg/ml concentrations of THC significantly decreased MV compared to vehicle at the 5- and 10-min timepoints and the 100 mg/ml concentration continued to suppress MV at the 15- and 20-min timepoints [Fig. 4A; F(33,66)=4.18, p<0.000001]. Suppression of MV (compared to vehicle) was not observed at later timepoints during the first 60 min of the session. This difference resulted primarily from the greater habituation and consequent decrease in MV that occurred in vehicle-treated male mice rather than an absolute difference in the effects of 100 and 300 mg/ml THC on MV. From 90-600 min post-exposure, THC effects on MV were not apparent in male mice (Fig. 4B).
In females, suppressant effects of the aerosolized 100 and 300 mg/ml concentrations of THC on MV (compared to aerosolized vehicle) were evident at 5-min after placement in the plethysmography chambers and, in contrast with males, persisted throughout the first 60 min of the session [Fig. 4C; dose X time interaction: F(33,66)=2.81, p=0.0002; main effect of dose: F(3,6)=72.25, p=0.00004]. In contrast, exposure to the lower 30 mg/ml THC concentration did not affect MV. Significant suppression of respiratory MV was short-lived, however, and did not carry over into the 90-600 min part of the session (Fig. 4D).
3.4.2. Telemetry (aerosol).
Following exposure to aerosolized THC, HR decreases were apparent in males, with significant decreases compared to vehicle observed starting at 10 min post-exposure for two THC concentrations (100 and 300 mg/ml) [Fig. 4E; F(33,66)=2.94, p=0.0001]. By 35- or 50-min post-exposure (for 300 and 100 mg/ml THC, respectively), THC-induced differences between HR compared to vehicle had dissipated. From 90 to 600 min post-exposure, HR was stable and was similar for vehicle and following exposure to any THC concentration (Fig. 4F).
In females, aerosolized THC (300 mg/ml) also decreased HR by 10 min after mice were placed into the plethysmography chambers (as compared to the first 5-min timepoint) [Fig. 4G; F(33,66)=2.00, p=0.008]. This decrease became significant compared to vehicle from 15 to 40 min post-exposure and at 100 mg/ml THC at a single timepoint (35 min post-exposure). Following these relatively short-lived decreases, HR stabilized in THC-treated mice and remained similar to vehicle levels for the remainder of the 600-min session (Fig. 4H).
4.0. Discussion
The safety and physiological impact of THC and novel cannabinoid receptor agonists can be difficult to assess, as many variables impact an individual’s response to increased cannabinoid receptor signaling. Age, sex, genetic and epigenetic factors, and delivery route can all modulate the duration and magnitude of downstream effects following THC administration [43]. Each of these factors can be controlled and individually tested in rodent models, and this study assesses the effect of sex and route of administration on THC-induced cardiopulmonary phenotypes. Miniaturization of telemetry devices and development of plethysmography procedures provides unprecedented opportunity for investigation of physiological functioning of freely moving rodents in real time. This study is among the first to investigate simultaneous measurement of respiration and cardiac functioning repeatedly over an extended period in the same animals.
Vehicle administration groups in this study represent the baseline impact of dose administration method and habituation to plethysmography chambers over time. Across routes of vehicle administration, there was an initial spike of respiration and heart rate that decreased across the first hour of data collection, which may be a result of habituation. The largest initial elevation of these phenotypes is seen in the animals’ first dose and exposure to plethysmography chambers (Figure 1). The initial habituation to the chambers may have affected the i.p. data, and particularly the i.p. vehicle data since that was the first exposure to the chambers. Given that i.p. was assessed first, any effects of habituation would have had a larger impact on i.p. than on other routes of administration.
Cannabinoid receptors are thought to impact respiration through both central and peripheral mechanisms [44]. Cannabinoid receptors, especially Cannabinoid 1 receptors (CB1R), are highly expressed in the brain, and recent work shows expression of cannabinoid receptors in the pre-Bötzinger complex of mice, a structure that produces the periodic drive for inspiration [45-47]. In the periphery, cannabinoid receptors also have a demonstrated role in the control of airway responsiveness [48, 49]. Short-term exposure to cannabis smoke in humans is most frequently associated with bronchodilation, though strong activation of cannabinoid signaling by synthetic cannabinoids may lead to respiratory depression [50-54]. Cannabis consumption is a concern in exacerbation of cardiovascular disease in humans, and increased cannabinoid receptor signaling is associated with a number of cardiovascular phenotypes, including tachycardia and decreased blood pressure [55-60].
In the present study, THC’s overall acute effect across routes of administration in male mice was to suppress respiration and heart rate. These results are consistent with results of previous research showing that intravenous THC administration, THC inhalation, and upregulation of endocannabinoid signaling each decrease heart rate and respiratory frequency in male rodents [61-63]. Central administration of cannabinoid receptor agonists in rats also leads to respiratory depression and decreased heart rate [64, 65]. The results herein demonstrate that this effect occurs independent of route of administration, although time course was notably different across routes of administration. Exposure to aerosolized THC led to strong acute impacts on cardiopulmonary phenotypes, whereas i.p. and s.c. injections had a delayed but longer-lasting impact. In agreement with data from rodent studies, THC decreases heart rate and respiratory minute volume in rhesus monkeys following intramuscular administration [66] and respiratory minute volume following subcutaneous administration [67], in cats following intravenous infusion of THC [68, 69], and in dogs following intravenous administration [70]. Intravenous injection of cannabinoid receptor agonists also reduces heart rate in rabbits [28, 71]. The direction of the THC-induced suppression of heart rate observed in animal studies is opposite of its tachycardiac effect observed in human users [55-60]. This species difference may be due to differing effective doses across human and animal studies [55, 72] or differences in distribution and involvement of cannabinoid receptors in the CNS and cardiovascular system [73].
Unlike other routes of administration, the p.o. data were not dose dependent, and this pattern was replicated across dependent measures. In particular, results from 3 and 10 mg/kg in the p.o. dose-effect curves for both sexes appear opposite of what would be expected based on the results of other routes of administration. The cause of this outcome is unknown. THC administered via the three other routes of administration (i.p., s.c., aerosol) produced dose-dependent decreases in heart rate and minute volume. The vehicle p.o. data represent the first time the mice were given a gavage dose. Stress associated with p.o. dosing may have contributed to the aberrant p.o. data as gavage-induced stress is a known potential confound in cardiac measures in mice [74]. Furthermore, there was generally more within-dose variability in the p.o. data compared to other routes of administration, and female p.o. data analyses indicated low power. Thus, the invasive nature of p.o. dosing and low power in the data may be factors in the lack of dose-dependent effects. One potential explanation for these unusual data is variability of THC absorption across animals based on when food and water were consumed before dose administration [75]. However, mice were fed at approximately the same time each day, and typically consumed their food ration relatively quickly after feeding, although some mice occasionally took a few hours to consume their food. There is not currently enough literature to understand how effects of the gavage dosing procedure may alter effects of cannabinoids on respiratory and cardiac parameters.
In female mice, the overall pattern of THC-induced decreases in respiration and heart rates is similar to that in males with one exception – effects of p.o. THC on heart rate. Females have a lower baseline heart rate compared to males, and p.o. THC-treated females do not demonstrate a THC-induced depression in heart rate relative to vehicle controls. The underlying cause of this difference in pattern in unknown, and there is no literature on whether female mice might have a low heart rate in response to stress as compared to males to our knowledge.
An important consideration for this work is that temperature and humidity within plethysmography chambers impact signal output [76-78], and cannabinoid receptor agonists induce dose-dependent hypothermia in mice [79]. Therefore, lack of control over humidity and temperature within the chamber is a limitation of this study. The impact of this hypothermic effect on plethysmography data and any differences in hypothermia across administration routes will be characterized in future studies.
5.0. Conclusion
Altogether, the work herein establishes the impact of dosing route on THC-induced heart rate and respiratory depression in male and female mice. To effectively interpret the body of literature surrounding THC dosing in animals, one must be cognizant of the impacts that sex, dose, and route of administration have on the magnitude and timing of response. This study highlights important differences in the timeline of cardiopulmonary response to THC following the most common preclinical routes of administration. Future studies may explore additional phenotypes, including the impact of dosing route and sex on THC-induced hypothermia.
Supplementary Material
Acknowledgements
The authors thank Shanequa Taylor, Nikita Pulley, and Kimberly Custer for excellent technical assistance. Statements and Declarations: Research was supported by U.S. National Institutes of Health / National Institute on Drug Abuse [grant numbers DA045003 and DA040460]. The funding source had no other role other than financial support. The authors have no relevant financial or non-financial interests to disclose. The datasets for the current study are available from the corresponding author on reasonable request.
References
- 1.Hasin DS, Shmulewitz D and Sarvet AL (2019). Time trends in US cannabis use and cannabis use disorders overall and by sociodemographic subgroups: a narrative review and new findings. Am. J. Drug Alcohol Abuse 45, 623–643, 10.1080/00952990.2019.1569668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S and Church JC (2016). Changes in cannabis potency over the last 2 fecades (1995-2014): Analysis of current data in the United States. Biol. Psychiatry 79, 613–619, 10.1016/j.biopsych.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cinnamon Bidwell L, YorkWilliams SL, Mueller RL, Bryan AD and Hutchison KE (2018). Exploring cannabis concentrates on the legal market: User profiles, product strength, and health-related outcomes. Addict Behav Rep 8, 102–106, 10.1016/j.abrep.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smart R, Caulkins JP, Kilmer B, Davenport S and Midgette G (2017). Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington state. Addiction 112, 2167–2177, 10.1111/add.13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA and ElSohly MA (2010). Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci 55, 1209–1217, 10.1111/j.1556-4029.2010.01441.x [DOI] [PubMed] [Google Scholar]
- 6.Modaresi F and Talachian K (2022). The Characteristics of Clinical Trials on Cannabis and Cannabinoids: A Review of Trials for Therapeutic or Drug Development Purposes. Pharmaceut. Med 36, 387–400, 10.1007/s40290-022-00447-7 [DOI] [PubMed] [Google Scholar]
- 7.Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C and Cone EJ (2017). Pharmacokinetic Profile of Oral Cannabis in Humans: Blood and Oral Fluid Disposition and Relation to Pharmacodynamic Outcomes. J. Anal. Toxicol 41, 83–99, 10.1093/jat/bkx012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naef M, Russmann S, Petersen-Felix S and Brenneisen R (2004). Development and pharmacokinetic characterization of pulmonal and intravenous delta-9-tetrahydrocannabinol (THC) in humans. J. Pharm. Sci 93, 1176–1184, 10.1002/jps.20037 [DOI] [PubMed] [Google Scholar]
- 9.Spindle TR, Cone EJ, Goffi E, Weerts EM, Mitchell JM, Winecker RE, Bigelow GE, Flegel RR and Vandrey R (2020). Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. 211, 107937, 10.1016/j.drugalcdep.2020.107937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sholler DJ, Zamarripa CA, Spindle TR, Martin EL, Kuntz D, Vandrey R and Grabenauer M (2022). Urinary Excretion Profile of Cannabinoid Analytes Following Acute Administration of Oral and Vaporized Cannabis in Infrequent Cannabis Users. J. Anal. Toxicol 46, 882–890, 10.1093/jat/bkac042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huestis MA, Blount BC, Milan DF, Newmeyer MN, Schroeder J and Smith ML (2019). Correlation of creatinine- and specific gravity-normalized free and glucuronidated urine cannabinoid concentrations following smoked, vaporized, and oral cannabis in frequent and occasional cannabis users. Drug Test Anal 11, 968–975, 10.1002/dta.2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabgay K, Waranuch N, Chaiyakunapruk N, Sawangjit R, Ingkaninan K and Dilokthornsakul P (2020). The effects of cannabis, cannabinoids, and their administration routes on pain control efficacy and safety: A systematic review and network meta-analysis. J. Am. Pharm. Assoc. (2003) 60, 225–234.e226, 10.1016/j.japh.2019.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Swortwood MJ, Newmeyer MN, Andersson M, Abulseoud OA, Scheidweiler KB and Huestis MA (2017). Cannabinoid disposition in oral fluid after controlled smoked, vaporized, and oral cannabis administration. Drug Test Anal 9, 905–915, 10.1002/dta.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemberger L, Weiss JL, Watanabe AM, Galanter IM, Wyatt RJ and Cardon PV (1972). Delta-9-tetrahydrocannabinol. Temporal correlation of the psychologic effects and blood levels after various routes of administration. N. Engl. J. Med 286, 685–688, 10.1056/nejm197203302861303 [DOI] [PubMed] [Google Scholar]
- 15.Sinclair J, Collett L, Abbott J, Pate DW, Sarris J and Armour M (2021). Effects of cannabis ingestion on endometriosis-associated pelvic pain and related symptoms. PLoS One 16, e0258940, 10.1371/journal.pone.0258940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiley JL, Taylor SI and Marusich JA (2021). Δ(9)-Tetrahydrocannabinol discrimination: Effects of route of administration in rats. Drug Alcohol Depend. 225, 108827, 10.1016/j.drugalcdep.2021.108827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, Nováková P, Šíchová K, Štefková K, Tylš F, Kuchař M and Páleníček T (2017). Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol 27, 1223–1237, 10.1016/j.euroneuro.2017.10.037 [DOI] [PubMed] [Google Scholar]
- 18.Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL and Fantegrossi WE (2014). In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ9-THC in mice: Inhalation versus intraperitoneal injection. Pharmacol. Biochem. Behav 124, 40–47, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baglot SL, Hume C, Petrie GN, Aukema RJ, Lightfoot SHM, Grace LM, Zhou R, Parker L, Rho JM, Borgland SL, McLaughlin RJ, Brechenmacher L and Hill MN (2021). Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Sci. Rep 11, 23990, 10.1038/s41598-021-03242-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welch SP, Thomas C and Patrick GS (1995). Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: possible mechanisms for interaction with morphine. J. Pharmacol. Exp. Ther 272, 310–321, [PubMed] [Google Scholar]
- 21.Borgen LA and Davis WM (1973). Vehicle and route of administration as parameters affecting operant behavioral effects of 9 -tetrahydrocannabinol. J. Pharm. Sci 62, 479–480, 10.1002/jps.2600620327 [DOI] [PubMed] [Google Scholar]
- 22.Manwell LA, Ford B, Matthews BA, Heipel H and Mallet PE (2014). A vapourized Δ(9)-tetrahydrocannabinol (Δ(9)-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J. Pharmacol. Toxicol. Methods 70, 112–119, 10.1016/j.vascn.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 23.Lichtman AH, Dimen KR and Martin BR (1995). Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl.) 119, 282–290, 10.1007/bf02246292 [DOI] [PubMed] [Google Scholar]
- 24.Lucas CJ, Galettis P and Schneider J (2018). The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 84, 2477–2482, 10.1111/bcp.13710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spindle TR, Martin EL, Grabenauer M, Woodward T, Milburn MA and Vandrey R (2021). Assessment of cognitive and psychomotor impairment, subjective effects, and blood THC concentrations following acute administration of oral and vaporized cannabis. Journal of psychopharmacology (Oxford, England) 35, 786–803, 10.1177/02698811211021583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spindle TR, Cone EJ, Herrmann ES, Mitchell JM, Flegel R, LoDico C, Bigelow GE and Vandrey R (2020). Pharmacokinetics of Cannabis Brownies: A Controlled Examination of Δ9-Tetrahydrocannabinol and Metabolites in Blood and Oral Fluid of Healthy Adult Males and Females. J. Anal. Toxicol 44, 661–671, 10.1093/jat/bkaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sholler DJ, Strickland JC, Spindle TR, Weerts EM and Vandrey R (2021). Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addict. Biol 26, e12968, 10.1111/adb.12968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samudre SS, Schneider JL, Oltmanns MH, Hosseini A, Pratap K, Loose-Thurman P, Allen RC, Williams PB, Lattanzio FA Jr. and Sheppard JD Jr. (2008). Comparison of topical and intravenous administration of WIN 55-212-2 in normotensive rabbits. Curr. Eye Res 33, 857–863, 10.1080/02713680802419724 [DOI] [PubMed] [Google Scholar]
- 29.Chaperon F, Soubrié P, Puech AJ and Thiébot MH (1998). Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl.) 135, 324–332, 10.1007/s002130050518 [DOI] [PubMed] [Google Scholar]
- 30.Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D and Morrison D (1988). Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl.) 96, 142–144, 10.1007/bf02431546 [DOI] [PubMed] [Google Scholar]
- 31.Lefever TW, Marusich JA, Antonazzo KR and Wiley JL (2014). Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacol. Biochem. Behav 118, 30–35, 10.1016/j.pbb.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valjent E and Maldonado R (2000). A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl.) 147, 436–438, 10.1007/s002130050013 [DOI] [PubMed] [Google Scholar]
- 33.Vlachou S, Nomikos GG, Stephens DN and Panagis G (2007). Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav. Pharmacol 18, 311–319, 10.1097/FBP.0b013e3282186cf2 [DOI] [PubMed] [Google Scholar]
- 34.Balster RL and Prescott WR (1992). Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci. Biobehav. Rev 16, 55–62, [DOI] [PubMed] [Google Scholar]
- 35.Wiley JL, Lowe JA, Balster RL and Martin BR (1995). Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J. Pharmacol. Exp. Ther 275, 1–6, [PubMed] [Google Scholar]
- 36.Aceto MD, Scates SM, Lowe JA and Martin BR (1996). Dependence on delta 9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J. Pharmacol. Exp. Ther 278, 1290–1295, [PubMed] [Google Scholar]
- 37.Lichtman AH, Fisher J and Martin BR (2001). Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol. Biochem. Behav 69, 181–188, 10.1016/s0091-3057(01)00514-7 [DOI] [PubMed] [Google Scholar]
- 38.Trexler KR, Eckard ML and Kinsey SG (2019). CB(1) positive allosteric modulation attenuates Δ(9)-THC withdrawal and NSAID-induced gastric inflammation. Pharmacol. Biochem. Behav 177, 27–33, 10.1016/j.pbb.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsou K, Patrick SL and Walker JM (1995). Physical withdrawal in rats tolerant to delta 9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur. J. Pharmacol 280, R13–15, 10.1016/0014-2999(95)00360-w [DOI] [PubMed] [Google Scholar]
- 40.Delaunois A, Dedoncker P, Hanon E and Guyaux M (2009). Repeated assessment of cardiovascular and respiratory functions using combined telemetry and whole-body plethysmography in the rat. J. Pharmacol. Toxicol. Methods 60, 117–129, 10.1016/j.vascn.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 41.National Research Council. (2011). Guide for the care and use of laboratory animals National Academies Press, Washington, D.C. [Google Scholar]
- 42.Wiley JL, Lefever TW, Glass M and Thomas BF (2019). Do you feel it now? Route of administration and Delta(9)-tetrahydrocannabinol-like discriminative stimulus effects of synthetic cannabinoids in mice. Neurotoxicology 73, 161–167, 10.1016/j.neuro.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitdumrongthum S and Trachootham D (2023). An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 28, 10.3390/molecules28062791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiese BM, Alvarez Reyes A, Vanderah TW and Largent-Milnes TM (2023). The endocannabinoid system and breathing. Front. Neurosci 17, 1126004, 10.3389/fnins.2023.1126004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glass M, Dragunow M and Faull RL (1997). Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299–318, 10.1016/s0306-4522(96)00428-9 [DOI] [PubMed] [Google Scholar]
- 46.Wiese BM, Liktor-Busa E, Levine A, Couture SA, Nikas SP, Ji L, Liu Y, Mackie K, Makriyannis A, Largent-Milnes TM and Vanderah TW (2021). Cannabinoid-2 Agonism with AM2301 Mitigates Morphine-Induced Respiratory Depression. Cannabis and cannabinoid research 6, 401–412, 10.1089/can.2020.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Negro CA, Funk GD and Feldman JL (2018). Breathing matters. Nat. Rev. Neurosci 19, 351–367, 10.1038/s41583-018-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calignano A, Kátona I, Désarnaud F, Giuffrida A, La Rana G, Mackie K, Freund TF and Piomelli D (2000). Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature 408, 96–101, 10.1038/35040576 [DOI] [PubMed] [Google Scholar]
- 49.Bozkurt TE (2019). Endocannabinoid System in the Airways. Molecules 24, 10.3390/molecules24244626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J and Fiellin DA (2007). Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch. Intern. Med 167, 221–228, 10.1001/archinte.167.3.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zwillich CW, Doekel R, Hammill S and Weil JV (1978). The effects of smoked marijuana on metabolism and respiratory control. Am. Rev. Respir. Dis 118, 885–891, 10.1164/arrd.1978.118.5.885 [DOI] [PubMed] [Google Scholar]
- 52.Alon MH and Saint-Fleur MO (2017). Synthetic cannabinoid induced acute respiratory depression: Case series and literature review. Respiratory medicine case reports 22, 137–141, 10.1016/j.rmcr.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jinwala FN and Gupta M (2012). Synthetic cannabis and respiratory depression. J. Child Adolesc. Psychopharmacol 22, 459–462, 10.1089/cap.2011.0122 [DOI] [PubMed] [Google Scholar]
- 54.Wong KU and Baum CR (2019). Acute Cannabis Toxicity. Pediatr. Emerg. Care 35, 799–804, 10.1097/pec.0000000000001970 [DOI] [PubMed] [Google Scholar]
- 55.Jones RT (2002). Cardiovascular system effects of marijuana. J. Clin. Pharmacol 42, 58s–63s, 10.1002/j.1552-4604.2002.tb06004.x [DOI] [PubMed] [Google Scholar]
- 56.Malit LA, Johnstone RE, Bourke DI, Kulp RA, Klein V and Smith TC (1975). Intravenous delta9-Tetrahydrocannabinol: Effects of ventilatory control and cardiovascular dynamics. Anesthesiology 42, 666–673, [PubMed] [Google Scholar]
- 57.DeFilippis EM, Bajaj NS, Singh A, Malloy R, Givertz MM, Blankstein R, Bhatt DL and Vaduganathan M (2020). Marijuana Use in Patients With Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol 75, 320–332, 10.1016/j.jacc.2019.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pacher P, Steffens S, Haskó G, Schindler TH and Kunos G (2018). Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat. Rev. Cardiol 15, 151–166, 10.1038/nrcardio.2017.130 [DOI] [PubMed] [Google Scholar]
- 59.Rezkalla S and Kloner RA (2019). Cardiovascular effects of marijuana. Trends Cardiovasc. Med 29, 403–407, 10.1016/j.tcm.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 60.Page RL 2nd, Allen LA, Kloner RA, Carriker CR, Martel C, Morris AA, Piano MR, Rana JS and Saucedo JF (2020). Medical Marijuana, Recreational Cannabis, and Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 142, e131–e152, 10.1161/cir.0000000000000883 [DOI] [PubMed] [Google Scholar]
- 61.Farra YM, Eden MJ, Coleman JR, Kulkarni P, Ferris CF, Oakes JM and Bellini C (2020). Acute neuroradiological, behavioral, and physiological effects of nose-only exposure to vaporized cannabis in C57BL/6 mice. Inhal. Toxicol 32, 200–217, 10.1080/08958378.2020.1767237 [DOI] [PubMed] [Google Scholar]
- 62.Iring A, Ruisanchez É, Leszl-Ishiguro M, Horváth B, Benkő R, Lacza Z, Járai Z, Sándor P, Di Marzo V, Pacher P and Benyó Z (2013). Role of endocannabinoids and cannabinoid-1 receptors in cerebrocortical blood flow regulation. PLoS One 8, e53390, 10.1371/journal.pone.0053390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmid K, Niederhoffer N and Szabo B (2003). Analysis of the respiratory effects of cannabinoids in rats. Naunyn Schmiedebergs Arch. Pharmacol 368, 301–308, 10.1007/s00210-003-0787-3 [DOI] [PubMed] [Google Scholar]
- 64.Padley JR, Li Q, Pilowsky PM and Goodchild AK (2003). Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br. J. Pharmacol 140, 384–394, 10.1038/sj.bjp.0705422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfitzer T, Niederhoffer N and Szabo B (2004). Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetised rats. Br. J. Pharmacol 142, 943–952, 10.1038/sj.bjp.0705874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO and Woods JH (1998). Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J. Pharmacol. Exp. Ther 286, 697–703, [PubMed] [Google Scholar]
- 67.Weed PF, Gerak LR and France CP (2018). Ventilatory-depressant effects of opioids alone and in combination with cannabinoids in rhesus monkeys. Eur. J. Pharmacol 833, 94–99, 10.1016/j.ejphar.2018.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doherty PA, McCarthy LE and Borison HL (1983). Respiratory and cardiovascular depressant effects of nabilone, N-methyllevonantradol and delta 9-tetrahydrocannabinol in anesthetized cats. J. Pharmacol. Exp. Ther 227, 508–516, [PubMed] [Google Scholar]
- 69.Graham JD and Li DM (1973). Cardiovascular and respiratory effects of cannabis in cat and rat. Br. J. Pharmacol 49, 1–10, [PMC free article] [PubMed] [Google Scholar]
- 70.Moss IR and Friedman E (1976). Delta9-tetrahydrocannabinol: depression of ventilatory regulation; other respiratory and cardiovascular effects. Life Sci. 19, 99–104, 10.1016/0024-3205(76)90379-9 [DOI] [PubMed] [Google Scholar]
- 71.Niederhoffer N and Szabo B (1999). Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol 126, 457–466, 10.1038/sj.bjp.0702337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchetti B, Bilel S, Tirri M, Arfè R, Corli G, Roda E, Locatelli CA, Cavarretta E, De Giorgio F and Marti M (2023). The Old and the New: Cardiovascular and Respiratory Alterations Induced by Acute JWH-018 Administration Compared to Δ(9)-THC-A Preclinical Study in Mice. Int. J. Mol. Sci 24, 10.3390/ijms24021631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards JR (2020). Mechanisms for the Risk of Acute Coronary Syndrome and Arrhythmia Associated With Phytogenic and Synthetic Cannabinoid Use. J. Cardiovasc. Pharmacol. Ther 25, 508–522, 10.1177/1074248420935743 [DOI] [PubMed] [Google Scholar]
- 74.Walker MK, Boberg JR, Walsh MT, Wolf V, Trujillo A, Duke MS, Palme R and Felton LA (2012). A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol. Appl. Pharmacol 260, 65–69, 10.1016/j.taap.2012.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lunn S, Diaz P, O'Hearn S, Cahill SP, Blake A, Narine K and Dyck JRB (2019). Human Pharmacokinetic Parameters of Orally Administered Δ(9)-Tetrahydrocannabinol Capsules Are Altered by Fed Versus Fasted Conditions and Sex Differences. Cannabis and cannabinoid research 4, 255–264, 10.1089/can.2019.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaui-Berlinck JG and Bicudo JE (1998). The signal in total-body plethysmography: errors due to adiabatic-isothermic difference. Respir. Physiol 113, 259–270, 10.1016/s0034-5687(98)00060-7 [DOI] [PubMed] [Google Scholar]
- 77.Lundblad LK, Irvin CG, Adler A and Bates JH (2002). A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol (1985) 93, 1198–1207, 10.1152/japplphysiol.00080.2002 [DOI] [PubMed] [Google Scholar]
- 78.Stucky F, Cazzaniga G, Aliverti A, Kayser B and Uva B (2020). Automating the correction of flow integration drift during whole-body plethysmography. Annu Int Conf IEEE Eng Med Biol Soc 2020, 5–8, 10.1109/embc44109.2020.9176170 [DOI] [PubMed] [Google Scholar]
- 79.Marusich JA, Gamage TF, Zhang Y, Akinfiresoye LR and Wiley JL (2022). In vitro and in vivo pharmacology of nine novel synthetic cannabinoid receptor agonists. Pharmacology, biochemistry, and behavior 220, 173467, 10.1016/j.pbb.2022.173467 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.