Abstract
Osteosarcoma (OS), also referred to as osteogenic sarcoma, is the most common primary malignant tumour affecting long bones, characterised by the proliferation of osteoblastic precursor cells and the production of osteoid or immature bone. OSs of the head and neck region have unique biology, exhibiting a clinical behaviour and a natural history that are distinct from OSs of the trunk and extremities. Similarly, their radiological appearance and histological growth pattern can be quite diverse proving to be a challenge to histopathologists to arrive at a diagnosis. Hence, OSs of the jaw remain enigmatic, and a number of difficulties related to their diagnosis and treatment are yet to be resolved. This article reports on a case of advanced OS of the mandible in a 45-year-old woman who came for the evaluation of swelling. This case illustrates the various modalities of diagnosis, such as radiology, histopathology and immunohistochemistry for the confirmation of the variants of OS, leading to an enormously improved quality of life by offering an improved opportunity for cure and treatment.
Keywords: Fibroblastic variant, mandible, osteosarcoma, pleomorphism, SATB2, spindle cells, storiform streaming pattern
INTRODUCTION
Osteosarcoma (OS) is a disease of mesenchymal cell origin characterised by the proliferation of osteoblastic precursors and the production of osteoid or immature bone. It usually affects the metaphysis of the long bones during skeletal growth in children and adolescents. Jaw osteosarcoma (JOS) is rare, accounting for only 6% of all OSs.[1]
In the initial phase of the disease, OS may present as an unremarkable, slowly progressing bony swelling, only to become overtly aggressive and malignant towards the latter phase of the disease, thus imposing a challenge to an accurate diagnosis.
It belongs to a family of lesions, which have considerable diversity in histologic features and grades. Histologically, OS is classified into three subtypes: osteoblastic, chondroblastic and fibroblastic. However, it may have various degrees of differentiation and produce various kinds of extracellular matrix, thus producing a histologic pattern that may vary significantly, not only from one case to another but also from area to area within the same case.[2]
OS of the jaw differs from the OS of the long bones in its biological behaviour, presenting a lower incidence of metastasis and a better prognosis with approximately 40% of 5-year survival rate as compared to 20% for non-jaw lesions.[3]
The purpose of this case report was to highlight the diagnostic difficulties encountered in a patient with OS, which affected the mandible and presented as swelling in the lower left third molar region.
CASE REPORT
A 45-year-old female patient reported to the Department of Oral and Maxillofacial Pathology of Guru Nanak Institute of Dental Sciences and Research, Kolkata, with a chief complaint of pain and swelling involving the lower left posterior region of jaw for the last four months. The patient presented with a history of extraction of a grade II mobile 36, four months ago, followed by numbness and tingling sensation in the anterior portion of the mandible along with pain and heaviness on the lower lip. Three months post-extraction, the patient observed a growth protruding out from the extraction socket that reached its maximum size of 3 × 3 cm within 15 days.
On clinical examination, extraorally there was a diffuse bony firm to hard swelling seen on the left lower third of the face, which is tender on palpation [Figure 1a and b]. The overlying skin appeared normal and free from the underlying structure, but the skin felt warm.
Figure 1.

Extraoral photograph of the patient revealing swelling on the left lower third of the face: (a) front profile and (b) side profile
Intraoral examination revealed a reddish pink-coloured, well-defined, exophytic growth protruding out of the extraction socket that was soft to firm and tender on palpation [Figure 2]. Another dome-shaped bony hard swelling was noted obliterating the left vestibular region with respect to 34 and 35, which was tender on palpation along with an expansion of buccal cortical plate. The overlying mucosal surface appeared normal.
Figure 2.

Intraoral photograph reveals smooth surfaced, reddish pink, well-defined swelling on the left lower molar region with respect to 37 protruding out of the extraction socket
An orthopantomogram [Figure 3] revealed ill-defined radiolucency involving the left mandibular body region extending from 35 to 38 along with marked thickening and enlargement of left interdental canal and mental foramen. A widening of periodontal ligament (PDL) space was also noted in relation to 36 and 38. The cone-beam computed tomography (CBCT) revealed the destruction of lingual cortical plate in relation to regional teeth (34, 35, 36 and 38) and abundance of calcific foci within the bony trabeculae of the affected teeth [Figure 4].
Figure 3.

Orthopantomogram revealed ill-defined radiolucency involving the left mandibular body region extending from 35 to 38 along with marked thickening and enlargement of the left interdental canal and mental foramen. A widening of PDL space was also noted in relation to 36 and 38
Figure 4.
(a and b) CBCT revealing destruction of the lingual cortical plate in relation to regional teeth (34, 35, 36 and 38) and an abundance of calcific foci within the bony trabeculae of the affected teeth
Based on the clinical and radiological features, our differential diagnosis included osteomyelitis, peripheral giant cell granuloma, peripheral ossifying fibroma and OS. The patient underwent the gold standard method of diagnosis. Following the incisional biopsy, the light microscopic features revealed the presence of parakeratotic stratified squamous surface epithelium supported by an underlying connective tissue stroma. The connective tissue stroma consisted of abundant bizarre-shaped cells following which immunohistochemistry (IHC) was performed. The spindle cells spotted in the histopathological section stained immunopositive for special AT-rich sequence-binding protein 2 (SATB2) [Figure 5] and immunonegative for pan-cytokeratin (AE1/AE3) [Figure 6], calponin, S100 protein [Figure 7] and smooth muscle actin [Figure 8] confirming the growth protruding out of the socket to be OS. Haematoxylin and eosin (H&E)-stained sections revealed the presence of numerous pleomorphic, hyperchromatic, abnormal osteoblast cells along with the areas of osteoid formation. Abnormal, pleomorphic, spindle-shaped fibroblast cells arranged in a storiform pattern with hyperchromatic nuclei were also noted in some areas [Figure 9]. The areas of mild degree of chronic inflammatory infiltration were also observed within the connective tissue stroma. The microscopic features established the fibroblastic variant of OS.
Figure 5.

IHC-stained immunopositive for SATB2: (a) 10x and (b) 40x
Figure 6.

IHC-stained immunonegative for pan-cytokeratin
Figure 7.

IHC-stained immunonegative for S100 protein
Figure 8.

IHC-stained immunonegative for smooth muscle actin
Figure 9.
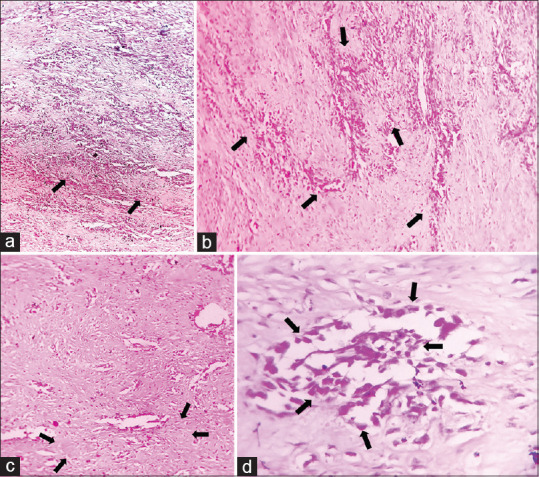
Histopathological image reveals the arrangement of the spindle cells with hyperchromatic nuclei in ‘streaming’ and ‘storiform’ patterns in the fibrovascular connective tissue stroma; the presence of pleomorphic and hyperchromatic spindle cells in the stroma: (a) 4x, (b) 4x, (c) 10x and (d) 40x
The patient was referred to the Department of Oral and Maxillofacial Surgery for further treatment where a hemimandibulectomy was performed extending from the left coronoid to the symphysis, and the patient was referred to a state general medical hospital for adjuvant radiotherapy and was advised for periodic follow-up. However, the patient did not comply with subsequent follow-up, post-surgery.
DISCUSSION
OS is primarily bone-producing malignant tumours arising most frequently in the long bones. Jaw OS usually occurs one to two decades later than that of OS of other regions. It has a bimodal age distribution, with a major peak in the second decade and a somewhat smaller peak after the age of 50.[2] Lesions are slightly more common in men. They occur with almost equal frequency in both jaws. The most common places of occurrence are the alveolar ridge and the body of maxilla and mandible.[4]
The exact cause of OS is unknown. However, a number of risk factors do exist. The role of rapid bone growth appears to predispose the patients to develop OS. There are cases of OS of the jaws, which are associated with other bone diseases, such as Paget’s disease, fibrous dysplasia, enchondromatosis and hereditary multiple exostosis, and with previous radiation therapy to the jaw region.[5]
Patients with OS often present with nonspecific complaints, such as pain in the affected area. Pain during sleep, enlarging mass and worsening pain without clear signs of infection or injury are particularly alarming signs. Physical examination findings may reveal a palpable mass, restricted joint movement, pain within the weight-bearing areas of the jaws or localised warmth and erythema.[6] In OS of jaw bones, swelling rather than pain is the most common finding[7] along with loosening of teeth, paraesthesia and nasal obstruction, which may also be recorded.[8] Most patients relate the occurrence of tumour to previous dental treatment, most commonly, dental extraction[9] and a rapid growth of tumour immediately after the tooth extraction, a phenomenon often shown by bone tumours.[10] The patient under discussion is a 45-year-old female who reported growth and pain in the left lower jaw after undergoing an extraction. Additionally, she complained of numbness and tingling sensation in the lower lip, which corroborated the clinical feature of OS mentioned by previous authors in different literature studies.
Radiographs typically demonstrate a poorly marginated or moth-eaten appearance of the bone with mixed amounts of cloudy mineralised matrix and areas of bone resorption. If the lesion has an associated soft-tissue mass, a discontinuous or broken periosteal reaction is traditionally spotted.[11] Generally, the tumour infringes the periosteum, leading to the formation of numerous thin irregular spicules of new bone, which may evolve outwards and perpendicular to the surface of the lesion producing the so-called sunray appearance. Lindquist et al.[12] reported that the widening of PDL and inferior dental canal, together with the sunburst effect, is almost pathognomonic of OS of jaw bone.[8] Codman’s triangle is formed due to the elevation of periosteum over the expanding tumour mass in a tent-like fashion.[13] Advanced imaging is best accomplished with magnetic resonance imaging (MRI) and should be performed for the entire bone where it will clearly demonstrate the extent of the bone marrow invasion, the presence and size of any soft-tissue mass and the relationship with surrounding vital structures.[14] The panoramic and CBCT views of the concerned patient revealed almost identical radiological features except for the very classical sunray pattern.
Laboratory findings are nondiagnostic, but high levels of alkaline phosphatase and lactate dehydrogenase have been shown to predict a poorer prognosis.[7]
The direct production of osteoid by malignant mesenchymal cells is a mandatory light microscopic finding. Along with the basic neoplastic osteoblast-like tumour cell, seven other tumour cell types have been reported, in particular:
Chondroblast-like
Fibroblast-like
Histiocyte-like
Myofibroblast
Osteoclast-like
Angioblast-like cells.[15]
Based on the predominant type of matrix, produced by the tumour, OSs are subclassified as follows:
Osteoblastic
Chondroblastic
Fibroblastic types.[7]
Osteoblastic osteosarcoma (OOS) is the most common histopathological variant where osteoid is present as lace-like networks between individual tumour cells undergoing calcification focally, whereas tumour cells lie in lacunae and form lobules in chondroblastic osteosarcoma (COS). The centre of the lobule has bony trabeculae producing a feathery appearance, and towards the periphery, the tumour becomes hypercellular.[9] The fibroblastic type reveals highly cellular areas of malignant, densely packed, atypical spindle-shaped cells with an enlarged nucleus recognised as fibroblasts arranged in a storiform pattern with a minimal amount of tumour osteoid.[15]
IHC is a cut above the rest, to rule out other sarcomas, such as malignant fibrous histiocytoma, fibrosarcoma and Ewing’s sarcoma (EWS). Caution needs to be exercised in the interpretation of the focal expression of a variety of markers, in particular S100, actin and epithelial membrane antigen found occasionally in otherwise typical OSs. Fibroblastic OS will be positive for vimentin and negative for S100, thus obviating the neural tumours.[2] SATB2 is a highly conserved nuclear matrix protein that acts as an attractive target for immunohistochemical identification of osteoblasts as it plays a critical role in osteoblast lineage commitment.[16] IHC did play a pivotal role in diagnosis. The storiform appearance of pleomorphic spindle-shaped fibroblast cells, minimum osteoid production, immunopositivity to SATB2 and immunonegativity to S100 [Figure 6a and b], calponin [Figure 7 a and b], smooth muscle actin [Figure 8 a and b] and pan-cytokeratin [Figure 9 a and b] in our case were thus very important characteristics of fibroblastic variant of OS.
Surgery represents the cornerstone of treatment for OSs. When dealing with this bone tumour, the multimodality therapy in the form of surgery plus neoadjuvant or adjuvant chemotherapy is the main protocol of treatment, but the best management of these cases still needs further research.[17] A wide radical resection is the treatment of choice for OS of jaws with clearance margins of 1.5–2 cm. In the mandible, the hemimandibulectomy is commonly preferred, which was carried out in this case followed by radiotherapy.
CONCLUSION
The rarity of the jaw lesions, their unusual presentation and diverse histopathological patterns make OS difficult to diagnose. The histological variation of OS cannot be identified by the clinical presentation or radiography. Hence, the microscopic examination of the tissue from all parts must always be performed as the type of OS may affect the treatment and prognosis. Identifying the pathogenesis responsible for the development of OS through molecular research may help in the development of newer diagnostic markers and help improve therapeutics, leading to a better prognosis and patient survival in the future. Late metastases may occur in ≥10 years after diagnosis, with no universally accepted stopping point for tumour surveillance.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors sincerely thank Prof. (Dr.) R R Paul Professor Emeritus (JIS University), Prof (Dr.) S K A Mahmud, Dr. Rudra Prasad Chatterjee, Dr. Neha Shah, Dr. Sanjeet Kr. Das, Dr. Swagata Gayen and Dr. Sudeshna Bagchi, for their immense help and relentless support.
REFERENCES
- 1.Bertin H, Gomez-Brouchet A, Rédini F. Osteosarcoma of the jaws:An overview of the pathophysiological mechanisms. Crit Rev Oncol Hematol. 2020;156:103126. doi: 10.1016/j.critrevonc.2020.103126. [DOI] [PubMed] [Google Scholar]
- 2.Desai D, Pandith S, Jeergal PA, Arathi K, Saini R. Fibroblastic variant of osteosarcoma:A challenge in diagnosis &management. Open Dent J. 2010;4:211–7. doi: 10.2174/1874210601004010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saify F, Jain S. Osteosarcoma of jaws:An insight into literature. Int J Orofac Biol. 2018;2:41–6. [Google Scholar]
- 4.Dixit AG, Parikh NJ. Osteosarcoma of jaw bone. J Indian Acad Oral Med Radiol. 2008;20:41. [Google Scholar]
- 5.Kalburge JV, Sahuji SK, Kalburge V, Kini Y. Osteosarcoma of mandible. J Clin Diagn Res. 2012;6:1597–9. doi: 10.7860/JCDR/2012/3922.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma:Prognostic importance and treatment implications. J Bone Joint Surg Am. 2002;84-A:49–57. [PubMed] [Google Scholar]
- 7.Chaudhary M, Chaudhary SD. Osteosarcoma of jaws. J Oral Maxillofac Pathol. 2012;16:233–8. doi: 10.4103/0973-029X.99075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angela C. Bone pathology. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and Maxillofacial Pathology. 3rd ed. Philadelphia: Saunders; 2009. pp. 660–4. [Google Scholar]
- 9.Nissanka EH, Amaratunge EA, Tilakaratne WM. Clinicopathological analysis of osteosarcoma of jaw bones. Oral Dis. 2007;13:82–7. doi: 10.1111/j.1601-0825.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 10.Adekeye EO, Chau KK, Edwards MB, Williams HK. Osteosarcoma of the jaws –A series from Kaduna, Nigeria. Int J Oral Maxillofac Surg. 1987;16:205–13. doi: 10.1016/s0901-5027(87)80132-7. [DOI] [PubMed] [Google Scholar]
- 11.Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther. 2016;3:221–43. doi: 10.1007/s40744-016-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindquist C, Teppo L, Sane J, Holmstrom T, Wolf J. Osteosarcoma of mandible:Analysis of 9 cases. J Oral Maxillofac Surg. 1986;44:759–64. doi: 10.1016/0278-2391(86)90149-7. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran R. Shafer's Textbook of Oral Pathology. Elsevier India. 2009 [Google Scholar]
- 14.Fox MG, Trotta BM. Osteosarcoma:Review of the various types with emphasis on recent advancements in imaging. Semin Musculoskelet Radiol. 2013;17:123–36. doi: 10.1055/s-0033-1342969. [DOI] [PubMed] [Google Scholar]
- 15.Peddana SK, Ramadas R, Cherian E, Thayalan D. Chondroblastic and fibroblastic osteosarcoma of the jaws:Report of two cases and review of literature. Indian J Dent Res. 2017;28:100–4. doi: 10.4103/ijdr.IJDR_792_14. [DOI] [PubMed] [Google Scholar]
- 16.Conner JR, Hornick JL. SATB 2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013;63:36–49. doi: 10.1111/his.12138. [DOI] [PubMed] [Google Scholar]
- 17.ElKordy MA, ElBaradie TS, ElSebai HI, Amin AA, KhairAlla SM. Osteosarcoma of the jaw:Challenges in the diagnosis and treatment. J Egypt Natl Cancer Inst. 2018;30:7–11. doi: 10.1016/j.jnci.2018.02.001. [DOI] [PubMed] [Google Scholar]



