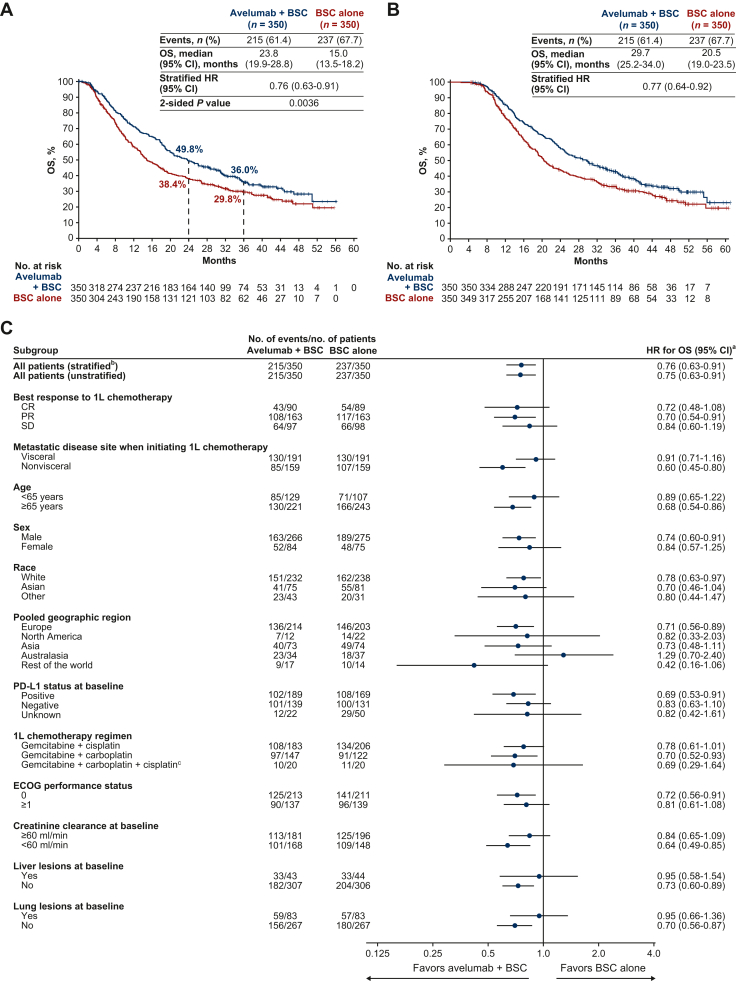
Figure 1.
OS in the overall population of the JAVELIN Bladder 100 trial (data cut-off, 4 June 2021). (A) OS measured from randomization at start of maintenance (i.e. after completion of chemotherapy; primary endpoint).69 (B) OS measured from start of first-line chemotherapy (exploratory analysis) in this selected trial population.70 (C) Subgroup analysis of OS (measured from randomization at start of maintenance).69
1L, first line; BSC, best supportive care; CI, confidence interval; CR, complete response; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; PD-L1, programmed death-ligand 1; OS, overall survival; PR, partial response; SD, stable disease.
aHRs and CIs were calculated using a Cox proportional hazards model.
bStratified by best response to 1L chemotherapy (CR or PR versus SD) and metastatic disease site when initiating 1L chemotherapy (visceral versus nonvisceral). Other HRs are unstratified.
cPatients who switched platinum regimens while receiving 1L chemotherapy.
Panels A and C adapted from Powles T, Park SH, Caserta C, et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up. J Clin Oncol. 2023;41(19):3486-3492. https://doi.org/10.1200/JCO.22.01792. © 2023 American Society of Clinical Oncology.