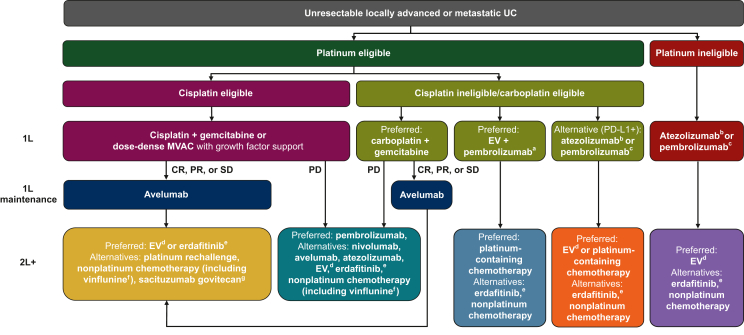
Figure 3.
Treatment sequencing in patients with advanced UC based on international treatment guidelines.6,10,13 Approval statuses and indications for each agent vary between countries; local labels must be consulted. Further details regarding FDA and EMA approvals are provided in footnotes. 1L, first line; 2L+, second line or later; CR, complete response; EMA, European Medicines Agency; EV, enfortumab vedotin; FDA, US Food and Drug Administration; MVAC, methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin; PD, progressive disease; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PR, partial response; SD, stable disease; UC, urothelial carcinoma.
aEV + pembrolizumab has received accelerated approval in the USA for the treatment of cisplatin-ineligible patients with advanced UC.59
bIn the 1L setting, atezolizumab is approved by the EMA, but not by the FDA, for the treatment of cisplatin-ineligible patients with advanced UC who have a PD-L1+ tumor.47,50
cIn the 1L setting, pembrolizumab is approved by the EMA for the treatment of cisplatin-ineligible patients with PD-L1+ advanced UC, and by the FDA for the treatment of patients with advanced UC who are not eligible for any platinum-containing chemotherapy (irrespective of PD-L1 status).48,51
dEV monotherapy has been approved by the EMA and FDA for the treatment of patients with advanced UC who have previously received treatment with platinum-containing chemotherapy and a PD-1 or PD-L1 inhibitor; in addition, EV monotherapy has been approved by the FDA for the treatment of cisplatin-ineligible patients who have received ≥1 prior line of therapy.59,94
eErdafitinib has been approved by the FDA for patients with advanced UC that has susceptible FGFR3 or FGFR2 genetic alterations and ≥1 line of prior platinum-containing chemotherapy; erdafitinib has not been approved by the EMA.95
fVinflunine has been approved by the EMA for patients with advanced UC after failure of prior platinum-containing therapy; vinflunine is not approved in the USA.96
gSacituzumab govitecan has been approved by the FDA for patients with advanced UC who have previously received treatment with platinum-containing chemotherapy and a PD-1 or PD-L1 inhibitor97; sacituzumab govitecan has not been approved by the EMA for patients with advanced UC.