Key Points
-
•
The murine and human fetal lung are a potential source of hemogenic endothelium.
-
•
Fetal lung–derived hemogenic endothelium relies on canonical EHT pathways.
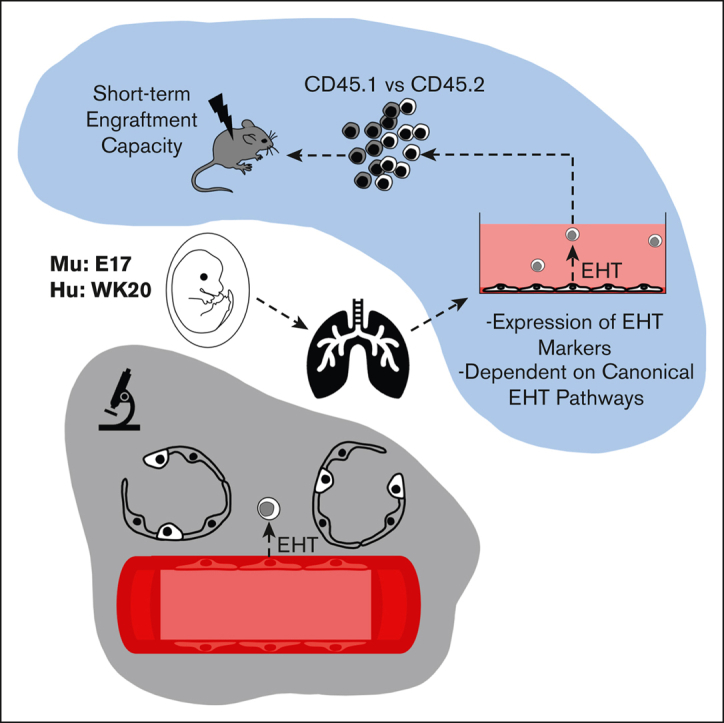
Visual Abstract
Abstract
Hemogenic endothelial cells (HECs) are specialized cells that undergo endothelial-to-hematopoietic transition (EHT) to give rise to the earliest precursors of hematopoietic progenitors that will eventually sustain hematopoiesis throughout the lifetime of an organism. Although HECs are thought to be primarily limited to the aorta-gonad-mesonephros (AGM) during early development, EHT has been described in various other hematopoietic organs and embryonic vessels. Though not defined as a hematopoietic organ, the lung houses many resident hematopoietic cells, aids in platelet biogenesis, and is a reservoir for hematopoietic stem and progenitor cells (HSPCs). However, lung HECs have never been described. Here, we demonstrate that the fetal lung is a potential source of HECs that have the functional capacity to undergo EHT to produce de novo HSPCs and their resultant progeny. Explant cultures of murine and human fetal lungs display adherent endothelial cells transitioning into floating hematopoietic cells, accompanied by the gradual loss of an endothelial signature. Flow cytometric and functional assessment of fetal-lung explants showed the production of multipotent HSPCs that expressed the EHT and pre-HSPC markers EPCR, CD41, CD43, and CD44. scRNA-seq and small molecule modulation demonstrated that fetal lung HECs rely on canonical signaling pathways to undergo EHT, including TGFβ/BMP, Notch, and YAP. Collectively, these data support the possibility that post-AGM development, functional HECs are present in the fetal lung, establishing this location as a potential extramedullary site of de novo hematopoiesis.
Introduction
Hematopoietic stem cells (HSCs) originate from a rare subpopulation of arterial endothelial cells known as HECs. Making up just 1% to 3% of the total endothelial cell population in the aorta-gonad-mesonephros (AGM), HECs are commonly thought to be confined to a small window of gestation between embryonic days 8 to 11 (E8-11) in mice and E27-40 in humans.1,2 Within this time window, HECs can also be found in other hematopoietic organs and vessels, including the yolk sac, placenta, and vitelline and umbilical arteries3, 4, 5, 6(p1),7,8 Some studies suggest that HECs may not be restricted to these hematopoietic organs and developmental window. Work from others suggests that functional HECs may also be found in the embryonic head around E10-E11 as well as in the perinatal chicken and murine bone marrow (BM).9,10 Collectively, these studies demonstrate that our spatiotemporal understanding of HECs remains limited and raises questions about the hematopoietic potential of other organs.
Recent studies have highlighted the presence and importance of various resident blood cell populations in the lung.11 In particular, the lung was demonstrated to be a reservoir of HSPCs, but the origins of this population of progenitors remain unknown.12, 13, 14 In utero mechanical stimuli have been shown to be an important environmental cue for both HEC and pulmonary development. More specifically, cyclic stretch-induced activation of Yes-activated protein (YAP) signaling in the AGM promotes endothelial-to-hematopoietic transition (EHT).15 Interestingly, rhythmic breathing movements begin occurring around E16 in fetal mice, and mechanical stimuli have a significant impact on fetal airway and alveolar epithelial development.16, 17, 18 Beyond mechanical signaling, cell-cell extrinsic signaling within the AGM niche is critical for EHT. The ventral wall of the dorsal aorta is the most common site of EHT because of its proximity to the underlying mesenchyme, which modulates EHT via Notch, BMP4, SHH, and Wnt pathways.3,19,20 All of these pathways are similarly important in pulmonary fetal development.21
Based on these parallels, we hypothesized that the fetal lung is another potential site of hemogenic endothelium with the functional capacity to produce hematopoietic progenitors. Employing fetal-lung explant cultures, we demonstrate that the murine and human fetal lungs are potential sources of HECs. Multipotent, fetal lung–derived HSPCs showed the canonical features of cells produced by EHT as determined by flow cytometry, single-cell transcriptomics, functional assays, and immunofluorescent histology. Although the methods used cannot definitively exclude the contribution of contaminant HSPCs to the observations described here, these findings highlight the fetal lung as another potential site of de novo hematopoiesis. This suggests that the lung may have a greater role in instructing tissue-specific hematopoiesis and/or overall hematopoietic development.
Methods
Tissue isolation and processing
Mouse
E17 timed-pregnant C57/BL6 mice were purchased from Jackson Laboratories. The fetal liver and lung were isolated from surrounding tissue by blunt dissection and set aside in a solution of 10% characterized fetal bovine serum in Hanks’ balanced salt solution (HBSS, Gibco). Using a 5 mL syringe fitted with a 16-gauge needle, the fetal liver and fetal lung were drawn up and expelled several times to physically dissociate the tissue. Samples were subsequently placed in a digest buffer containing HBSS, 1 mg/mL DNase I (Sigma), and 0.5 mg/mL LiberaseTM (Sigma). This digest mixture was placed on a rocker at 37°C for 30 minutes to 1 hour. After digestion, lung samples were filtered and resuspended in RBC lysis for 5 minutes at 37°C, then washed, filtered, and resuspended in HSPC medium.
Human
All fetal samples were within the age range between 19 and 24 weeks after conception. Lung lobes were dissected away from the main airways and minced with a scalpel before being placed in a digest buffer containing HBSS, 1 mg/mL DNase I (Sigma), and 0.5 mg/mL LiberaseTM (Sigma). This digest mixture was placed on a rocker at 37°C for 30 minutes to 1 hour. After digestion, lung samples were filtered and resuspended in RBC lysis for 5 minutes at 37°C, then washed, filtered, and resuspended in HSPC medium.
Explant cultures
Isolated cells suspended in HSPC medium were plated onto Matrigel-coated plates. HSPC medium was made up of StemPro-34 serum-free medium, 50 μg/mL ascorbic acid, 400 nM monothioglycerol, 100 μg/mL Primocin, 2 mM L-glutamine, and the following human or murine growth factors: 50 ng/mL vascular endothelial growth factor A, 100 ng/mL basic fibroblast growth factor, 100 ng/mL stem cell factor, 100 ng/mL FMS-related tyrosine kinase ligand (FLT3LG), 100 ng/mL thrombopoietin, and 100 ng/mL interleukin-6. On the third day of all cultures, media was aspirated, rinsed once with PBS, and fresh media was applied.
All animal housing and experimental procedures were approved by the Boston University School of Medicine Institutional Animal Care and Use Committee. Work involving human tissue samples was approved by Partners Human Research Committee (protocol #2016P001106).
Results
Murine fetal-lung explant cultures produce HSPCs
To assess the functional capacity of potential lung HECs to produce blood progenitors, cells isolated from murine E17 lungs were plated onto Matrigel-coated plates in an adapted hematopoietic differentiation medium, termed HSPC medium.22 This serum-free medium was formulated to support the final stages of the specification of HECs into HSPCs. A schematic of this culture protocol is provided in supplemental Figure 3C. The primary rationale for choosing E17 was that, (1) this is during the window that fetal breathing movements are occurring,16 and (2) E17 is a time point that is distant from the AGM EHT window (E8-11)1; thus helping to minimize contamination from AGM-EHT–derived progenitors.
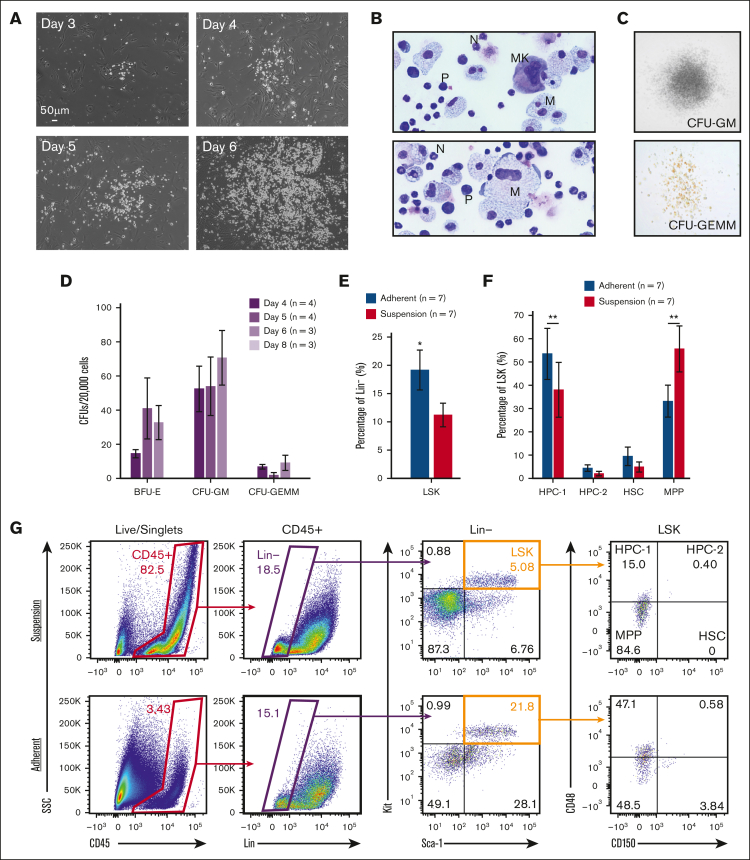
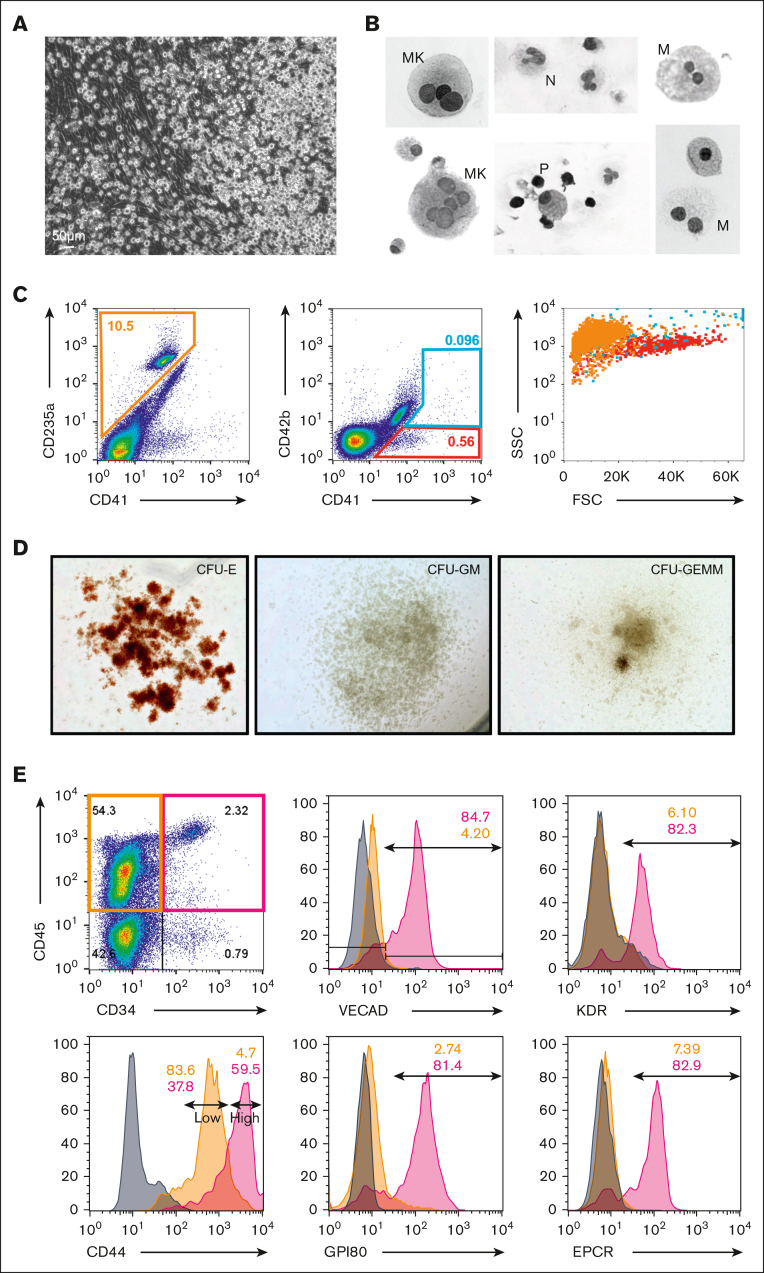
In contrast to fetal liver explants, where immediate expansion of floating cells is observed because of an expanding hematopoietic progenitor population, fetal-lung explants initially developed a robust adherent layer. Discrete clusters of suspension cells are observed by day 3, which expand to robust colonies between days 4 to 6 (Figure 1A). To visually determine cell identity based on their morphology, cytospins of day 6 suspension cells were performed, which revealed cells with a progenitor-like morphology as well as various differentiated hematopoietic cells, including macrophages/monocytes, neutrophils, and megakaryocytes (Figure 1B). This diversity of hematopoietic cells suggests that hematopoietic progenitors are arising from these cultures. To examine this, suspension cells were functionally assessed for progenitor potential by the MethoCult assay. Days 4 to 6 suspension cells showed similar potential to form all types of colony-forming units, but this potential was no longer detected at day 8 (Figure 1C-D). Progenitor phenotyping was further assessed by flow cytometric assessment of the broad murine HSPC markers Lin-/Sca+/Kit+ (LSK) and the SLAM markers CD48 and CD150. A significantly greater fraction of CD45+ hematopoietic cells isolated from the adherent cell layer were LSK-HSPCs and hematopoietic progenitor cell 1 (HPC-1) progenitors (Figure 1E-G). Collectively, these data suggest that the adherent layer of cells from fetal-lung explants gave rise to HSPCs.
Figure 1.
Murine fetal-lung explant cultures produce HSPCs. (A) Images of a single position between days 3 to 6 of a murine fetal-lung explant culture. (B) Cytospin of fetal lung–derived suspension cells. Images are annotated for cell type: MK, megakaryocyte; N, neutrophil; M, monocyte/macrophage; P, progenitor. (C) Images of representative colonies from a colony-forming unit assay. (D) Colony counts from colony-forming unit assays performed with day 4, 5, 6, and 8 bulk unsorted suspension cells. Each n represents a set of colony assays with at least 3 technical replicates from a distinct biological replicate. (E-G) Flow cytometric assessment of LSK and SLAM marker–defined HSPC populations. Each n represents a biological replicate with a minimum of 3 technical replicates per experiment. Suspension represents cells that were floating in media, and adherent represents cells that were collected after treatment with Accutase. Error bars represent standard error. ∗P < .05, ∗∗P < .01.
Murine fetal-lung explants exhibit the dynamics of EHT
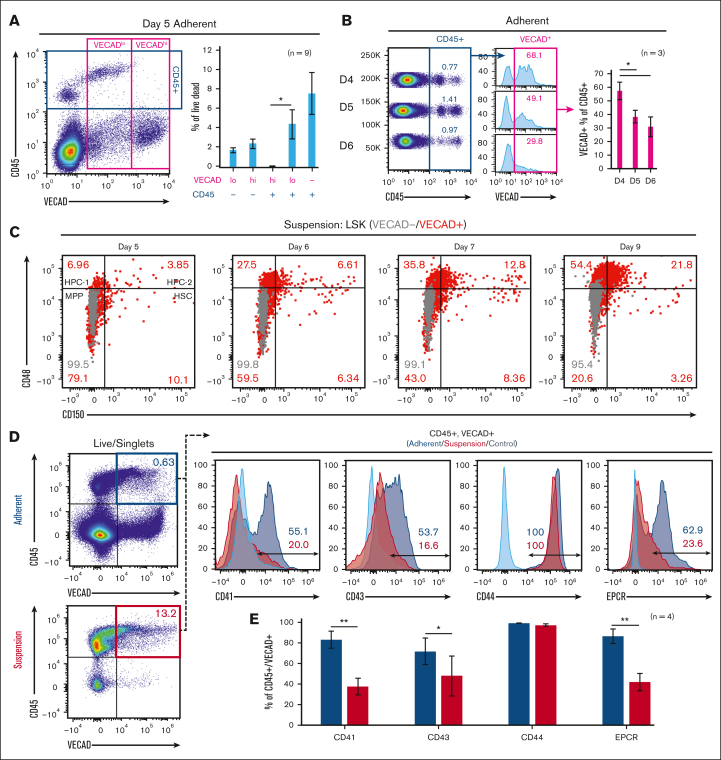
Time-lapse capture of live explant cultures from days 3 to 6 showed adherent cells transitioning to suspension cells that further divide to form robust colonies of suspension cells (supplemental Videos 1-3). These observations closely mimic AGM explant cultures and in vitro–based hematopoietic differentiations, showing the transition of HECs into hematopoietic cells.23, 24, 25, 26 The resultant pre-HSPCs that are birthed from AGM-derived HECs retain some endothelial markers and are marked by coexpression of the endothelial marker vascular endothelial cadherin (VECAD) and the broad hematopoietic marker CD45.27 Fetal-lung explants gave rise to VECAD+/CD45+ cells, with a significantly greater fraction of VECADlo/CD45+ vs VECADhi/CD45+ events (Figure 2A). The fraction of CD45+ cells that were VECAD+ also decreased with successive days of culture (Figure 2B). Together, this suggests that as cells transition and express the broad hematopoietic marker CD45, the expression of VECAD is downregulated and reduces over time. Time-matched fetal livers, which house HSPCs but not HECs,28 were cultured under the same conditions and showed a significantly lower fraction of VECAD+/CD45+ cells (supplemental Figure 1A,C). This suggests that fetal lung–derived VECAD+/CD45+ pre-HSPCs are not a result of from the expansion of preexisting progenitors. SLAM marker–defined HSCs were predominantly present among the VECAD+ suspension cells (Figure 2C). With time, a gradual reduction in the fraction of VECAD+ HSCs was observed, which coincided with the expansion of the more lineage-restricted HPC-1 and HPC-2 populations.
Figure 2.
Murine fetal-lung explants exhibit the dynamics of EHT. (A) Flow cytometric assessment of VECAD and CD45 expression on day 5 fetal-lung explants. (B) CD45 and VECAD expression across days 4 to 6. (C) Assessment of SLAM marker–defined HSPC populations stratified by VECAD expression across multiple days in culture. (D-E) Assessment of pre-HSC and EHT markers on VECAD+/CD45+–defined progenitors and separated by adherent vs suspension cell populations. Each n represents a biological replicate with a minimum of 3 technical replicates per experiment. Error bars represent standard error. ∗P < .05, ∗∗P < .01.
Beyond VECAD, successive staging from a pro-HSC to a pre-HSC phenotype is marked by the expression of CD41 and CD43, respectively.29 Endothelial protein C receptor (EPCR) and CD44 have also been previously demonstrated as markers of pre-HSPCs.29, 30, 31 Fetal lung–derived VECAD+/CD45+ cells showed expression of CD41, CD43, CD44, and EPCR (Figure 2D). There was a significant reduction in CD41, CD43, and EPCR expression as these cells transitioned into a suspension state (Figure 2E). The intensity of EPCR expression also decreased as VECAD+/CD45- cells matured away from an endothelial signature (supplemental Figure 1E). In contrast, CD41 expression transiently peaked at the VECAD+/CD45+ pre-HSPC stage. Under these experimental conditions, however, no notable changes in CD44 expression were observed regardless of physical or maturational state. We also observed significantly less expression of the pre-HSC markers CD41 and EPCR on fetal liver VECAD+/CD45+ cells, suggesting that, in contrast to those from the fetal lung, these cells are likely not pre-HSPCs (supplemental Figure 1B,D).
Murine fetal lung–derived HSPCs are capable of long-term engraftment
Fetal-lung explant–derived HSPCs were functionally assessed in a competitive transplantation assay to determine their capacity for engraftment. A schematic of the experimental design can be found in supplemental Figure 2. In brief, VECAD+/EPCR+ progenitors were sorted from C57BL6 CD45.2 fetal-lung explants and cotransplanted with C57BL6 CD45.1 BM progenitor cells into lethally irradiated CD45.1 recipient mice. This sorting strategy was used to ensure the enrichment of endothelial-derived cells with a greater capacity for engraftment, as VECAD and EPCR are both endothelial markers, and EPCR is a marker that has been previously shown to enhance enrichment of engraftable HSCs.32, 33, 34 Engraftment efficiency was determined by assessing the presence of CD45.2+ transplanted cells in peripheral blood at 1-month intervals. CD45.2+ cells derived from fetal-lung explants were detected 4 months after transplantation, suggesting that fetal lung–derived HSPCs have the capacity for short-term engraftment.
Single-cell transcriptomic mapping of murine fetal lung EHT
To map the developmental trajectory of cells produced in the murine fetal-lung explant model system and to illustrate the repertoire of cells involved in the process, single-cell RNA sequencing (scRNA-seq) was performed. The optimal time window for EHT in our model was determined to be from days 4 to 6 with the peak of CD45+/VECADlo cells on day 5 (supplemental Figure 3A). Because cells that have recently undergone EHT transiently retain expression of VECAD, adherent cells were collected into a single-cell suspension and sorted for VECAD+ to enrich for both endothelial cells and cells undergoing transition (supplemental Figure 3B). To ensure the capture of progenitor cells as well as differentiated cells that have downregulated VECAD after EHT, suspension cells were collected for sequencing but left unsorted. The single cell capture and processing was performed using the Chromium 10× Genomics platform and subsequently sequenced using the Illumina NextSeq 2000 platform. A schematic of the sequencing methodology as well as cell capture numbers and read depth can be found in supplemental Figure 3C-D.
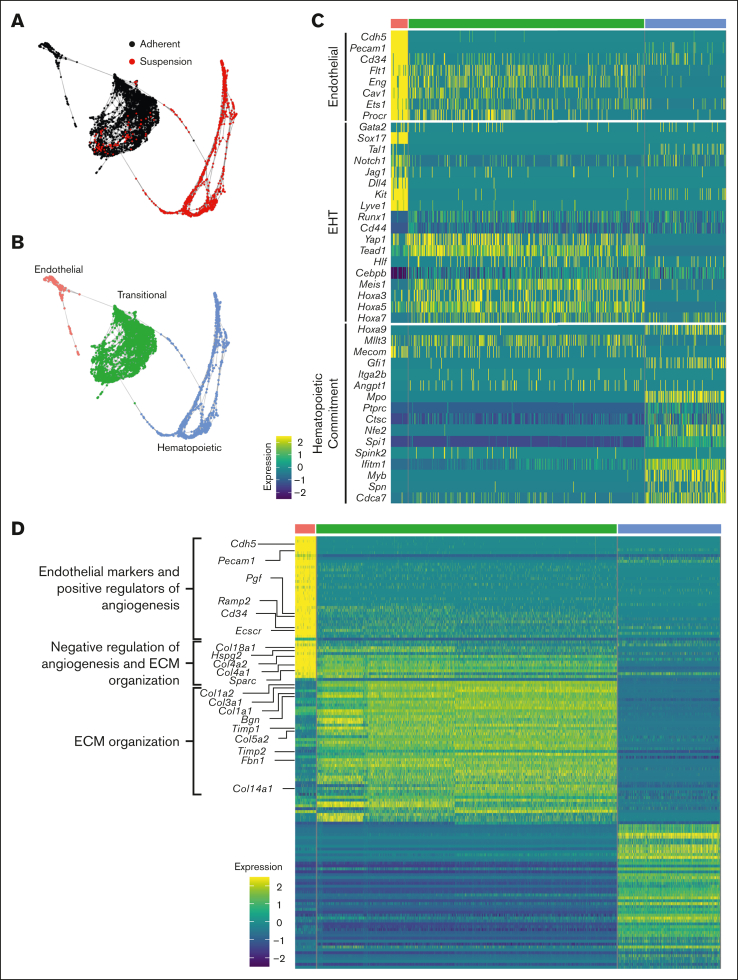
SPRING analysis revealed a trajectory of cells transitioning from an adherent to a suspension state through 3 distinct clusters: endothelial, transitional, and hematopoietic (Figure 3A-B). These clusters were annotated based on their top differentially expressed genes and by cross referencing their transcriptomic profile with gene ontology (supplemental Figures 4 and 5B). The transitional cluster was so named as the overall transcriptomic profile was somewhere between the more clearly defined endothelial and hematopoietic clusters. Furthermore, the current literature has yet to definitively define the transitional EHT cell. To further assess and identify these clusters, our data set was harmonized with a previously published data set of scRNA-seq analysis of cells isolated from the E9.5-E11 embryonic dorsal aorta.35 The transitional and HSPC clusters defined here aligned most closely with Zhu et al’s hemogenic endothelial and intra-aortic clusters, respectively, by Pearson correlation analysis (supplemental Figure 5). A harmonized UMAP projection shows the transitional cluster closely opposed to the clusters actively undergoing EHT (pre-H, hemogenic endothelial, and intra-aortic clusters) (supplemental Figure 6).
Figure 3.
Single-cell transcriptomic mapping of murine fetal lung EHT. (A-B) SPRING plot trajectory of EHT clusters. (C) Heat map of supervised gene expression analysis of endothelial, EHT, and hematopoietic commitment markers. (D) Heat map of unsupervised analysis of the top 50 differentially expressed genes. Representative gene ontology analysis is highlighted here along with the associated genes enriched in these biological processes. Full DGE analysis can be found under supplemental data. A natural log transformation was done to normalization gene expression. Further details regarding scRNA-seq data processing is described in supplemental Methods.
Supervised gene expression analyses of canonical endothelial markers showed robust expression and subsequent downregulation in the endothelial and transitional populations, respectively (Figure 3C). Analysis of genes previously described as important during EHT, many of which were recently reviewed,3 highlighted the expression of these key markers at a discrete stage of EHT. For example, Notch1, Jag1, Dll4, and Sox17 were largely isolated to the endothelial cell stage. In contrast, Yap1 and Tead1 demonstrated sustained expression through the transitional cell stage. These results are in agreement with other reports demonstrating that Notch1 and Sox17 are critical in the early patterning of HECs, but their continued expression throughout EHT can be restrictive.36,37 In contrast, Yap was previously shown to be critical in the maintenance of EHT but not its initiation.15 Finally, expression of Lyve1 was predominantly expressed during the endothelial stage, which suggests the production of a definitive wave of hematopoiesis38(p1). Dynamic expression of Hoxa genes was also previously reported to have a significant impact on hematopoietic development. Hoxa3, Hoxa5, and Hoxa7 were expressed during the transitional stage, which is consistent with prior reports showing that these genes are important in HSPC specification.39,40 In contrast, Hoxa9 was largely limited to the hematopoietic stage, which is in line with a recent report that identified a 6 gene HSC signature (RUNX1+ HOXA9+ MLLT3+ MECOM+ HLF+ SPINK2+).41 However, expression of this full 6 gene profile was not appreciable in the hematopoietic cluster.
As cells transitioned to hematopoietic commitment, they began expressing key pre-HSPCs genes, including Cdca7, Myb, Gfi1, and Spn (CD43) (Figure 3C). Expression of Cdca7 was previously demonstrated to be isolated to pre-HSC populations found within AGM intra-aortic hematopoietic clusters.42 Myb is critical in HSC maintenance and proliferation,43 Gfi1 is critical in mediating the loss of an endothelial identity44(p1); and Spn (CD43) is a known marker of early pre-HSCs.29,45 Notably, expression of these genes was primarily isolated to early progenitor clusters and decreased along the trajectory of maturation (supplemental Figure 7A), which is in agreement with other reports showing that their downregulation is necessary to allow for competent differentiation.
Complementary to these supervised analyses, unsupervised analysis of the top 50 differentially expressed genes showed that transitional cells retain the expression of genes involved in the downregulation of angiogenesis as they differentiate away from an endothelial fate (Figure 3D; supplemental Figure 7B and supplemental Data). This coincided with the upregulation of genes critical to extracellular matrix organization as these cells undergo a physical transition from an adherent to a suspension state. In agreement with these findings, others have demonstrated that the emergence of hematopoietic cells from HECs relies on the Runx1-mediated upregulation of genes involved in extracellular matrix organization, cell adhesion, and cell migration46(p1). Many of the same genes, including Runx1, were upregulated predominantly within the transitional cluster (Figure 3C-D).
Murine fetal lung EHT is functionally reliant on canonical developmental pathways
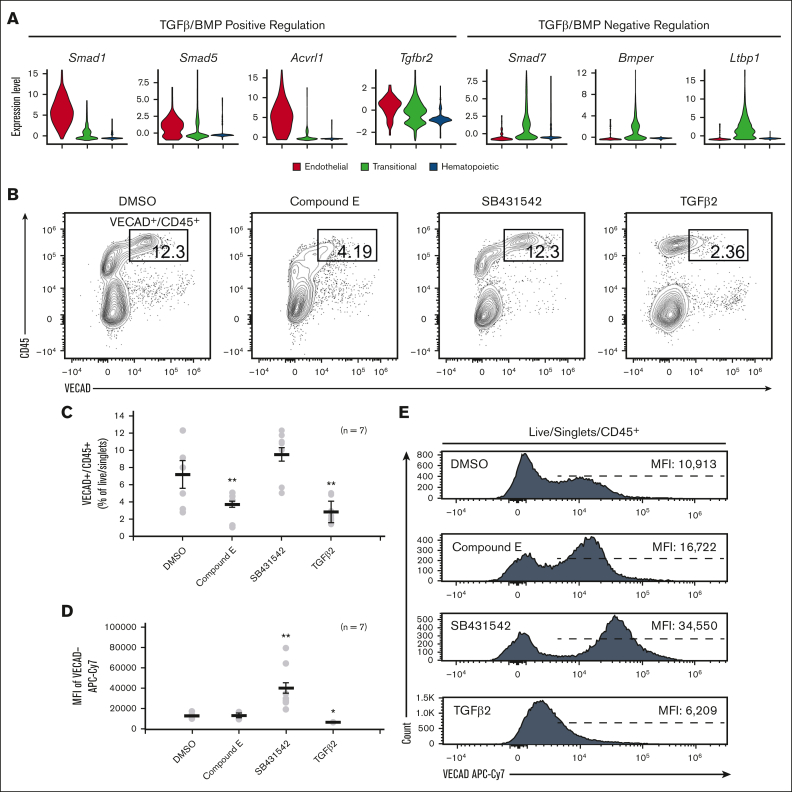
To functionally assess the dependence of fetal lung EHT on Notch signaling, the Notch inhibitor, compound E, was applied to explant cultures. Application of compound E led to a significant reduction in VECAD+/CD45+ pre-HSPCs (Figure 4B-C). This is in line with other reports demonstrating that in vitro Notch inhibition and in vivo transgenic knockout of Notch1 specifically block EHT, but the proliferation, differentiation, and maintenance of HSPCs are conserved.36,47, 48, 49, 50
Figure 4.
Fetal lung EHT is functionally reliant on canonical developmental pathways. (A) Violin plots of expression of TGFβ/BMP pathways genes. (B) Representative flow cytometry plots demonstrating the change in CD45+/VECAD+ progenitors after treatment with the Notch inhibitor compound E, the TGFβ inhibitor SB431542, and recombinant TGFβ2. (C) Counts of CD45+/VECAD+ progenitors after treatment. (D) Average MFI of VECAD expression after treatment. (E) Representative histograms of VECAD expression after treatment. Error bars represent standard deviation. ∗∗P < .01 compared with DMSO control. Each n represents a biological replicate with a minimum of 3 technical replicates per experiment.
In addition to the Notch family genes described previously, gene expression analysis demonstrated that fetal lung HECs exhibit the dynamic regulation of TGFβ/BMP pathways required for EHT. Similar to Notch, TGFβ/BMP pathways are important in the early patterning of HECs, but their subsequent inhibition is required for EHT.37,51, 52, 53 Expression of key mediators of TGFβ/BMP signaling, including Smad1, Smad5, Acvrl1, and Tgfbr2, peaked at the endothelial stage and was progressively downregulated throughout EHT (Figure 4A). Driving this downregulation was the concurrent upregulation of the TGFβ/BMP inhibitors Smad7, Bmper, and Ltbp1.
To assess the dependence of TGFβ signaling in fetal lung EHT, the TGFβ receptor inhibitor SB431542 (20μM) and recombinant TGFβ2 (50nM) were applied to explant cultures. Application of recombinant TGFβ2 led to a significant reduction in VECAD+/CD45+ pre-HSPCs (Figure 4B-C). In contrast, SB431542 did not significantly affect the production of VECAD+/CD45+ pre-HSPCs. However, SB431542 induced a significant increase in the intensity of VECAD expression, whereas TGFβ2 caused a reduction (Figure 4D-E). Although others have shown that SB431542 treatment can enhance the production of HSPCs,37,54 these findings suggest that there is already sufficient inhibition from the upregulation of TGFβ/BMP inhibitors discussed previously.
Human fetal-lung explants undergo EHT to produce HSPCs
To understand if this phenomenon is conserved in humans, cells collected from human fetal lungs isolated from fetuses aged between 19 and 24 weeks after conception were cultured under the same conditions described above. Similar to murine fetal-lung explants, human fetal-lung explants initially developed an adherent layer and gave rise to suspension cells between days 7-10 (Figure 5A). Cytospins and flow cytometry of suspension cells showed progenitor-like cells and various differentiated cell types, including megakaryocytes, red blood cells, monocytes/macrophages, and neutrophils (Figure 5B-C). Suspension cells assessed by the MethoCult assay also showed colony-forming unit capacity (Figure 5D). These data collectively suggest that human fetal-lung explants are giving rise to HSPCs capable of multilineage differentiation.
Figure 5.
Human fetal-lung explants undergo EHT to produce HSPCs. (A) Image of a human fetal-lung explant culture showing a robust population of floating hematopoietic cells against a background of adherent cells. (B) Cytospin of human fetal lung suspension cells. (C) Flow cytometric assessment of differentiated erythrocyte and megakaryocyte populations. (D) Images of representative colonies from a colony-forming unit assay. (E) Assessment of pre-HSC and EHT markers on CD34+/CD45+–defined progenitors vs CD34–/CD45+–differentiated hematopoietic cells.
To assess whether these suspension cells were derived via EHT, flow cytometric assessment of endothelial and HSPC markers was performed on human fetal-lung explants (Figure 5E). Human HSPCs were broadly defined by the coexpression of the progenitor/endothelial marker CD34 and the hematopoietic marker CD45. Most fetal-lung–derived CD34+/CD45+ progenitors coexpressed VECAD, which was downregulated as these cells differentiated and lost expression of CD34. In addition, fetal-lung–derived HSPCs expressed the HSPC markers KDR, GPI80, CD44, and EPCR. A reduction in the expression of these pre-HSPC/HSPC markers coincided with the loss of CD34 expression. Notably, human fetal-lung–derived HSPCs were enriched with a distinct CD44-high population, which others have reported is a marker of type II pre-HSPCs found within the intra-aortic hematopoietic clusters of the AGM.30
Human fetal lung EHT is directed toward the interstitium
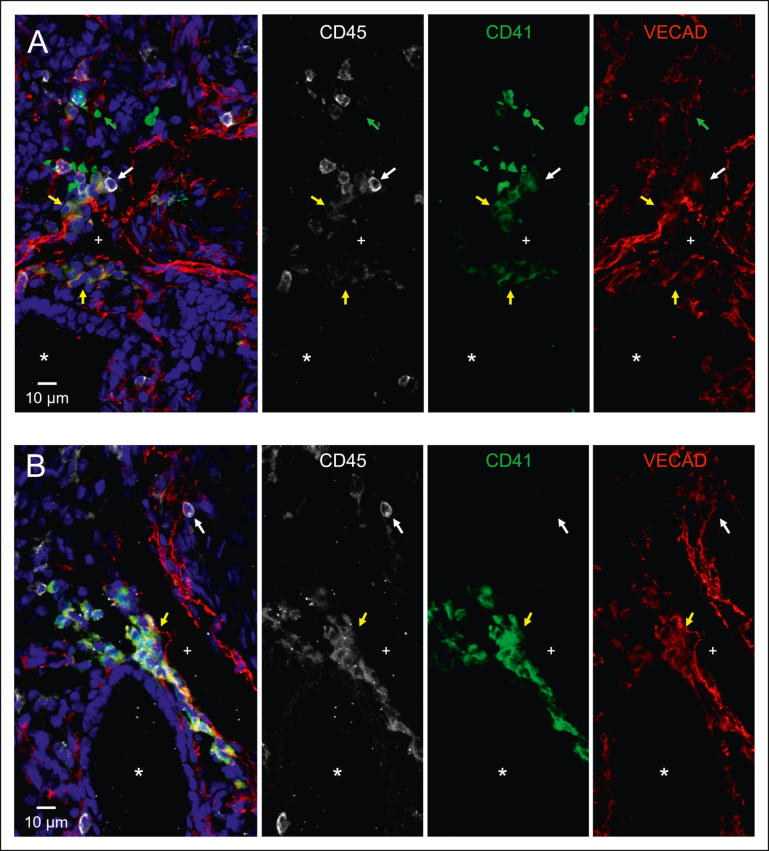
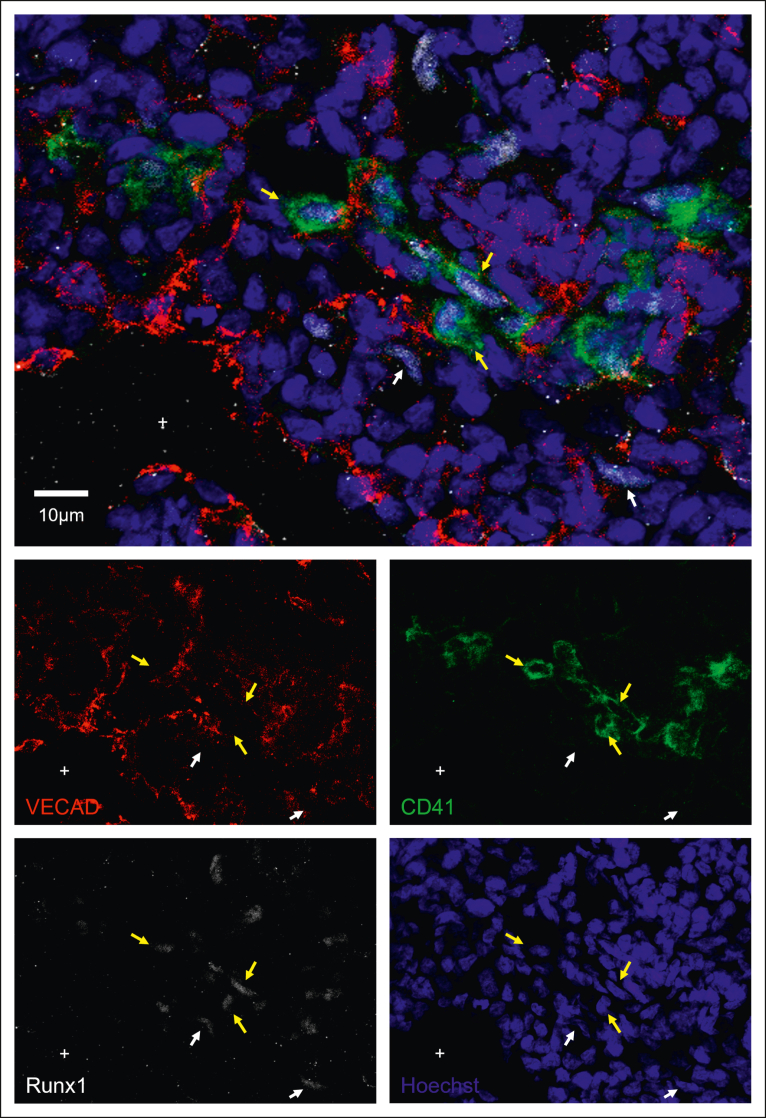
Intravascular hematopoietic clusters are a common histological hallmark of EHT occurring within the AGM and can be found in other organs that exhibit EHT, including the placenta and the umbilical and vitelline arteries.8 To investigate whether fetal lung EHT can occur in vivo, we performed immunofluorescent staining of fixed-frozen human fetal lung sections. Immunofluorescent analysis revealed distinct regions of hematopoietic cell clusters coexpressing the broad hematopoietic marker CD45, the endothelial marker VECAD, and the pre-HSC marker CD41 (Figure 6A-B; supplemental Figure 8). These regions of EHT also show positive nuclear staining for the critical EHT transcriptional regulator Runx1 (Figure 7; supplemental Figure 9). These data, combined with additional staining for E-cadherin (supplemental Figure 10), suggest that the emergence of these clusters is oriented away from the vascular lumen and projecting into the interstitial spaces of the fetal lung. Notably, this is in direct contrast to other described sites of hemogenic endothelium wherein EHT is directed toward the vascular lumen to allow produced cells to enter circulation. This suggests that the fate of these budding hematopoietic cells is preferentially aligned toward residing in the lung.
Figure 6.
Immunofluorescent staining shows that in situ fetal lung EHT is directed toward the interstitium. Twelve μm sections of a fixed frozen postconception week-20 human fetal lung stained with the broad hematopoietic marker CD45, the EHT marker CD41, the endothelial marker VECAD, and the nuclear stain Hoechst. The lumen of a large vessel lined with VECAD+ staining is highlighted with a “+,” and the lumen of a VECAD– developing epithelial space is highlighted with a “∗.” (A-B) Cells coexpressing VECAD, CD41, and CD45 are highlighted with yellow arrows showing developing hematopoietic clusters oriented toward the lung interstitium. Anucleate platelets single-positive for CD41 are highlighted with green arrows. Differentiated hematopoietic cells only positive for CD45 are highlighted with white arrows. A wide-field image of section (A), a section including ECAD staining, and a control stain is included in supplemental Figures 8, 10, and 11.
Figure 7.
Immunofluorescent staining shows Runx1 expression during in situ fetal-lung EHT. Twelve μm sections of a fixed frozen postconception week-20 human fetal lung stained with the EHT marker CD41, the nuclear EHT marker Runx1, the endothelial marker VECAD, and the nuclear stain Hoechst. Cells that are Runx1+ and VECAD+ are highlighted with a white arrow, whereas cells that are Runx1+ and CD41+ are highlighted with a yellow arrow. The lumen of a large vessel lined with VECAD+ staining is highlighted with a “+.” Additional Runx1 staining can be found in supplemental Figure 9.
Discussion
Although not defined as a hematopoietic organ, the lung houses many resident blood cells that carry out both broad and tissue-specific hematopoietic functions. Immune surveillance in the lung is carried out via unique resident immune cells, including dendritic cells, T cells, B cells, alveolar macrophages, interstitial macrophages, innate lymphoid cells, and natural killer cells.11,55 Supporting these classical immune cells are lung-resident megakaryocytes, which have a unique immune phenotype in addition to carrying out platelet biogenesis.56,57 Finally, the murine lung has been demonstrated to house HSPCs.12, 13, 14 Complementary to these prior findings, the evidence provided here suggests that the murine and human fetal lungs are a potential source of functional HECs capable of giving rise to HSPCs. This suggests that the developing lung may have a more direct role in hematopoiesis than previously thought.
Although we have demonstrated the possible existence of fetal pulmonary HECs, the physiological significance of de novo hematopoiesis occurring outside of the AGM and in the lung remains unclear. The potential need for the fetal lung to have an alternative source of blood may stem from the fact that most blood is shunted away from the fetal lung because oxygenation is provided via the mother. Thus, the developing murine lung only receives about 16% of the total circulating blood.58 The de novo generation of blood in the fetal lung may act as an additional in situ source of essential blood products for organogenesis. This is an important consideration when accounting for the role of macrophages and platelets in both organogenesis and angiogenesis.59, 60, 61, 62 Notably, the origins of many lung-resident blood cells are not fully understood, but to date, the origins of resident macrophages are the most well studied.
Lung macrophages are derived from 3 distinct developmental waves originating from the yolk sac, fetal liver, and BM.63 Each wave persists into adulthood and occupies unique niches within the lung.64 Recent work also suggests that such developmentally and phenotypically distinct subsets of macrophages may also extend to the heart, liver, kidney, and brain.65 These findings demonstrate the complex diversity seen throughout hematopoietic development and how each phase individually makes significant physiological contributions. As such, the differentiation capacity of lung HECs requires further investigation to determine whether lung HECs contribute to the in situ development of lung hematopoietic populations.
Although often discussed as the precursor to HSCs, HECs also give rise to other blood progenitors. A subset of placental HEC lineages traced by Hoxa13 preferentially form placental macrophages, termed Hofbauer cells, that remain solely in the placenta.66 This finding also suggests that differentiation capacity and bias may already be predetermined at the HEC stage, which others have also proposed. THBS1 marks a subset of human embryonic stem cell–derived endothelial cells that are biased toward megakaryopoiesis.67 Ly6a vs Tek expression marks HECs with HSC vs EMP potential, respectively.68 CXCR4 expression differentiates AGM-derived HECs with HSC vs MPP potential.69
Others have described the presence of functional HSCs in E16 murine fetal lungs. However, LSK progenitors only make up, on average, about 0.02% of the total fetal lung cell isolate, compared with 0.75% in the adult BM.14 This low number of HSPCs suggests that the role of fetal lung HECs may not contribute significantly to the progenitor cell pool and instead is skewed toward the immediate production of differentiated cell populations. The work presented here also suggests this, as we show marked production of HPC-1 progenitors and a transcriptomic profile that is inconsistent with recent reports of an HSC signature.41 However, we show that fetal-lung–derived HSPCs do have some capacity for short-term engraftment, and work from others has shown that the lung does have the capacity to repopulate blood populations.12 More work needs to be done to determine not only the capacity of lung-derived progenitors but also the circumstances in which lung HECs make a significant contribution to hematopoiesis. Given that HSPCs, although rare, are present in the fetal lung, an alternative hypothesis to be considered is the possibility that preexisting lung HSPCs are expanding in the explant cultures used here. This would suggest instead that the fetal lung may be a reservoir and/or a site for the expansion of HSPCs. Current available models for tracing endothelium are of limited utility in this regard because of the significant expression of endothelial genes in hematopoietic progenitors during fetal development.70 As such, application of these methods would not sufficiently discriminate preexisting HSPCs from lung HECs, especially during the embryonic migration of HSPCs exiting the fetal liver.71 Lung-specific HEC tracing models would be the optimal modality to definitively demonstrate the existence of lung-specific HECs, HSPCs, or both.
Although more in vivo work is required to determine the developmental trajectory and potency of lung HECs, the contributions of hematopoiesis that are not reliant on long-term HSCs are important to consider. Indeed, most embryonic hematopoiesis is maintained by a pool of yolk sac–derived erythromyeloid progenitors (EMPs) that reside in the fetal liver.72,73 EMPs give rise to most of the tissue-resident macrophages that persist into adulthood,74 and embryonic erythrocyte production is sustained primarily from EMPs.75 In addition to EMPs, transient HSCs that do not persist into adulthood have also been demonstrated to be involved in the establishment of the developing hematopoietic system. Tie2-Cre lineage tracing studies suggest that fetal HSCs that contribute to the establishment of the developing hematopoietic system differentiate rapidly, which is in stark contrast to adult long-term HSCs that are relatively quiescent.76 Studies using the Flk2-Cre tracer model (FlkSwitch) also demonstrate the existence of a transient population of HSCs with a lymphoid bias.77,78 Although fulfilling the classic definition of an HSC based on their capacity for long-term multilineage reconstitution, these transient HSCs are only present from E10.5 until P14.
Notably, the fetal lung HECs described here were isolated from E17 mice, which is outside of the window of EHT within the AGM (E9.5-11.5). Work from Yvernogeau et al also suggests that EHT can occur beyond this AGM window within the perinatal BM.10 They noted that perinatal sources of hematopoiesis occur at a time when HSC expansion in the fetal liver has stopped (∼E17) and the BM niche is still maturing to support the long-term residency of adult HSCs. Later-stage EHT in the lung may thus aid in minimizing the need for long-term HSC-dependent hematopoiesis during early development.
Niche interactions are another critical component of driving EHT.4,79 Induction of key developmental pathways via secreted ligands is spatially organized within the AGM and influences the potency of resultant progenitors.20 Replicating these conditions is thus critical in simulating EHT in an in vitro setting. Most other methods for ex vivo HEC cultures are reliant on the use of a supportive feeder cell layer, such as OP9 cells or immortalized AKT endothelial cells, to instruct EHT.47,80 Notably, fetal-lung explants undergo EHT without such feeders, which suggests that the lung independently has the cellular makeup to support EHT. However, further investigation is required to determine the cell-specific interactions of the in vivo microenvironment where EHT would occur and how the lung niche instructs local HEC development.
Altogether, the findings described here suggest that the fetal lung is a potential source of HECs, a population that is also conserved in humans. Given the diversity in HEC development and the significance of each distinct wave of hematopoiesis, the physiological influence of lung HEC development in vivo needs further investigation. Expanding our overall understanding of HEC location and timing will also aid toward the goal of fully understanding the biological cues required for the development of functional HECs. Such findings can eventually be harnessed for the common goal of producing putative HSCs and functionally mature hematopoietic cells from pluripotent stem cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
This work was supported by National Institutes of Health (NIH) training grant for Hematology 5T32HL7501-36, the NIH Predoctoral National Research Service Award (NRSA) for MD/PhD Fellowships (1F30HL154552-01), and internal funds were provided by Boston University School of Medicine. The authors thank Brian R. Tilton of the Boston University Flow Cytometry Core Facility, Yuriy Alekseyev and Ashley LeClerc from the Boston University Microarray and Sequencing Resource Core, and Michael T. Kirber from the Boston University Cellular Imaging Core for technical support.
Authorship
Contribution: A.K.Y. performed experimental design, data collection, data analysis, interpretation of data, and manuscript preparation; G.J.M. performed and supervised experimental design, interpretation of data, and manuscript preparation; C.V.-M. and J.L.-V. performed the bioinformatic/computational analysis and supported the experimental design and manuscript preparation; P.B. and F.W. performed bioinformatic/computational analysis and supported manuscript preparation; and A.C.B, K.V., T.W.D., A.B.Y., V.V., G.M., and A.B.B. assisted with data collection and manuscript preparation.
Footnotes
Single-cell RNA sequencing data were made publicly available on GEO: accession: GSE197290, Token: wvqjuouqdtmrtuh. All other requests can be directed to the corresponding author, George J. Murphy (gjmurphy@bu.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Wu Y, Hirschi KK. Regulation of hemogenic endothelial cell development and function. Annu Rev Physiol. 2021;83(1):17–37. doi: 10.1146/annurev-physiol-021119-034352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lievre F, Peault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87(1):67–72. [PubMed] [Google Scholar]
- 3.Lange L, Morgan M, Schambach A. The hemogenic endothelium: a critical source for the generation of PSC-derived hematopoietic stem and progenitor cells. Cell Mol Life Sci. 2021;78(9):4143–4160. doi: 10.1007/s00018-021-03777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heck AM, Ishida T, Hadland B. Location, location, location: how vascular specialization influences hematopoietic fates during development. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.602617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Ferkowicz MJ, Johnson SA, Shelley WC, Yoder MC. Endothelial cells in the early murine yolk sac give rise to CD41-expressing hematopoietic cells. Stem Cells Dev. 2005;14(1):44–54. doi: 10.1089/scd.2005.14.44. [DOI] [PubMed] [Google Scholar]
- 6.Chen MJ, Yokomizo T, Zeigler B, Dzierzak E, Speck NA. Runx1 is required for the endothelial to hematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodes KE, Gekas C, Wang Y, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2(3):252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yzaguirre AD, Speck NA. Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites. Dev Dyn. 2016;245(10):1011–1028. doi: 10.1002/dvdy.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Lan Y, He W, et al. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell. 2012;11(5):663–675. doi: 10.1016/j.stem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Yvernogeau L, Gautier R, Petit L, et al. In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium. Nat Cell Biol. 2019;21(11):1334–1345. doi: 10.1038/s41556-019-0410-6. [DOI] [PubMed] [Google Scholar]
- 11.Ardain A, Marakalala MJ, Leslie A. Tissue-resident innate immunity in the lung. Immunology. 2020;159(3):245–256. doi: 10.1111/imm.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefrançais E, Ortiz-Muñoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Summer R, Kotton DN, Liang S, Fitzsimmons K, Sun X, Fine A. Embryonic lung side population cells are hematopoietic and vascular precursors. Am J Respir Cell Mol Biol. 2005;33(1):32–40. doi: 10.1165/rcmb.2005-0024OC. [DOI] [PubMed] [Google Scholar]
- 14.Hillel-Karniel C, Rosen C, Milman-Krentsis I, et al. Multi-lineage lung regeneration by stem cell transplantation across major genetic barriers. Cell Rep. 2020;30(3):807–819.e4. doi: 10.1016/j.celrep.2019.12.058. [DOI] [PubMed] [Google Scholar]
- 15.Lundin V, Sugden WW, Theodore LN, et al. YAP regulates hematopoietic stem cell formation in response to the biomechanical forces of blood flow. Dev Cell. 2020;52(4):446–460.e5. doi: 10.1016/j.devcel.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niblock MM, Perez A, Broitman S, Jacoby B, Aviv E, Gilkey S. In utero development of fetal breathing movements in C57BL6 mice. Respir Physiol Neurobiol. 2020;271 doi: 10.1016/j.resp.2019.103288. [DOI] [PubMed] [Google Scholar]
- 17.Tang Z, Hu Y, Wang Z, et al. Mechanical forces program the orientation of cell division during airway tube morphogenesis. Dev Cell. 2018;44(3):313–325.e5. doi: 10.1016/j.devcel.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Wang Z, Chu Q, Jiang K, Li J, Tang N. The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Dev Cell. 2018;44(3):297–312.e5. doi: 10.1016/j.devcel.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Canu G, Ruhrberg C. First blood: the endothelial origins of hematopoietic progenitors. Angiogenesis. 2021;24(2):199–211. doi: 10.1007/s10456-021-09783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souilhol C, Gonneau C, Lendinez JG, et al. Inductive interactions mediated by interplay of asymmetric signalling underlie development of adult haematopoietic stem cells. Nat Commun. 2016;7(1) doi: 10.1038/ncomms10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141(3):502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung A, Zulick E, Skvir N, et al. Notch and aryl hydrocarbon receptor signaling impact definitive hematopoiesis from human pluripotent stem cells: definitive hematopoiesis in pluripotent stem cells. STEM CELLS. 2018;36(7):1004–1019. doi: 10.1002/stem.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yvernogeau L, Gautier R, Khoury H, et al. An in vitro model of hemogenic endothelium commitment and hematopoietic production. Development. 2016;143(8):1302–1312. doi: 10.1242/dev.126714. [DOI] [PubMed] [Google Scholar]
- 24.Ohta R, Sugimura R, Niwa A, Saito MK. Hemogenic endothelium differentiation from human pluripotent stem cells in a feeder- and xeno-free defined condition. JoVE (Journal of Visualized Experiments) 2019;(148) doi: 10.3791/59823. [DOI] [PubMed] [Google Scholar]
- 25.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 26.Eilken HM, Nishikawa SI, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 27.Taoudi S, Gonneau C, Moore K, et al. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3(1):99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Fantin A, Tacconi C, Villa E, Ceccacci E, Denti L, Ruhrberg C. KIT is required for fetal liver hematopoiesis. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.648630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottersbach K. Endothelial-to-haematopoietic transition: an update on the process of making blood. Biochem Soc Trans. 2019;47(2):591–601. doi: 10.1042/BST20180320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oatley M, Bölükbası ÖV, Svensson V, et al. Single-cell transcriptomics identifies CD44 as a marker and regulator of endothelial to haematopoietic transition. Nat Commun. 2020;11(1):586. doi: 10.1038/s41467-019-14171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fidanza A, Stumpf PS, Ramachandran P, et al. Single-cell analyses and machine learning define hematopoietic progenitor and HSC-like cells derived from human PSCs. Blood. 2020;136(25):2893–2904. doi: 10.1182/blood.2020006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanuytsel K, Villacorta-Martin C, Lindstrom-Vautrin J, et al. Multi-modal profiling of human fetal liver hematopoietic stem cells reveals the molecular signature of engraftment. Nat Commun. 2022;13(1):1103. doi: 10.1038/s41467-022-28616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balazs AB, Fabian AJ, Esmon CT, Mulligan RC. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2006;107(6):2317–2321. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramaniam A, Talkhoncheh MS, Magnusson M, Larsson J. Endothelial protein C receptor (EPCR) expression marks human fetal liver hematopoietic stem cells. Haematologica. 2019;104(2):e47–e50. doi: 10.3324/haematol.2018.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Q, Gao P, Tober J, et al. Developmental trajectory of prehematopoietic stem cell formation from endothelium. Blood. 2020;136(7):845–856. doi: 10.1182/blood.2020004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumano K, Chiba S, Kunisato A, et al. Notch1 but not notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18(5):699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 37.Vargel Ö, Zhang Y, Kosim K, et al. Activation of the TGFβ pathway impairs endothelial to haematopoietic transition. Sci Rep. 2016;6(1) doi: 10.1038/srep21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee LK, Ghorbanian Y, Wang W, et al. LYVE1 marks the divergence of yolk sac definitive hemogenic endothelium from the primitive erythroid lineage. Cell Rep. 2016;17(9):2286–2298. doi: 10.1016/j.celrep.2016.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng ES, Azzola L, Bruveris FF, et al. Differentiation of human embryonic stem cells to HOXA+ hemogenic vasculature that resembles the aorta-gonad-mesonephros. Nat Biotechnol. 2016;34(11):1168–1179. doi: 10.1038/nbt.3702. [DOI] [PubMed] [Google Scholar]
- 40.Dou DR, Calvanese V, Sierra MI, et al. Medial HOXA genes demarcate haematopoietic stem cell fate during human development. Nat Cell Biol. 2016;18(6):595–606. doi: 10.1038/ncb3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvanese V, Capellera-Garcia S, Ma F, et al. Mapping human haematopoietic stem cells from haemogenic endothelium to birth. Nature. 2022;604(7906):534–540. doi: 10.1038/s41586-022-04571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guiu J, Bergen DJM, De Pater E, et al. Identification of Cdca7 as a novel Notch transcriptional target involved in hematopoietic stem cell emergence. J Exp Med. 2014;211(12):2411–2423. doi: 10.1084/jem.20131857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greig KT, Carotta S, Nutt SL. Critical roles for c-Myb in hematopoietic progenitor cells. Semin Immunol. 2008;20(4):247–256. doi: 10.1016/j.smim.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Lancrin C, Mazan M, Stefanska M, et al. GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood. 2012;120(2):314–322. doi: 10.1182/blood-2011-10-386094. [DOI] [PubMed] [Google Scholar]
- 45.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108(6):2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lie-A-Ling M, Marinopoulou E, Li Y, et al. RUNX1 positively regulates a cell adhesion and migration program in murine hemogenic endothelium prior to blood emergence. Blood. 2014;124(11):e11–e20. doi: 10.1182/blood-2014-04-572958. [DOI] [PubMed] [Google Scholar]
- 47.Hadland BK, Varnum-Finney B, Poulos MG, et al. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros–derived hematopoietic stem cells. J Clin Invest. 2015;125(5):2032–2045. doi: 10.1172/JCI80137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J Clin Invest. 2011;121(3):1207–1216. doi: 10.1172/JCI43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maillard I, Koch U, Dumortier A, et al. Canonical Notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2(4):356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancini SJC, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105(6):2340–2342. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C, Lv J, He Q, et al. Inhibition of endothelial ERK signalling by Smad1/5 is essential for haematopoietic stem cell emergence. Nat Commun. 2014;5(1):3431. doi: 10.1038/ncomms4431. [DOI] [PubMed] [Google Scholar]
- 52.Monteiro R, Pinheiro P, Joseph N, et al. Transforming growth factor β drives hemogenic endothelium programming and the transition to hematopoietic stem cells. Dev Cell. 2016;38(4):358–370. doi: 10.1016/j.devcel.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGarvey AC, Rybtsov S, Souilhol C, et al. A molecular roadmap of the AGM region reveals BMPER as a novel regulator of HSC maturation. J Exp Med. 2017;214(12):3731–3751. doi: 10.1084/jem.20162012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C, Tang X, Sun X, et al. TGFβ inhibition enhances the generation of hematopoietic progenitors from human ES cell-derived hemogenic endothelial cells using a stepwise strategy. Cell Res. 2012;22(1):194–207. doi: 10.1038/cr.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barker KA, Etesami NS, Shenoy AT, et al. Lung-resident memory B cells protect against bacterial pneumonia. J Clin Invest. 2021;131(11) doi: 10.1172/JCI141810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pariser DN, Hilt ZT, Ture SK, et al. Lung megakaryocytes are immune modulatory cells. J Clin Invest. 2021;131(1) doi: 10.1172/JCI137377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeung AK, Villacorta-Martin C, Hon S, Rock JR, Murphy GJ. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020;4(24):6204–6217. doi: 10.1182/bloodadvances.2020002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou YQ, Cahill LS, Wong MD, Seed M, Macgowan CK, Sled JG. Assessment of flow distribution in the mouse fetal circulation at late gestation by high-frequency Doppler ultrasound. Physiol Genomics. 2014;46(16):602–614. doi: 10.1152/physiolgenomics.00049.2014. [DOI] [PubMed] [Google Scholar]
- 59.Neo WH, Lie-A-Ling M, Fadlullah MZH, Lacaud G. Contributions of embryonic HSC-independent hematopoiesis to organogenesis and the adult hematopoietic system. Front Cell Dev Biol. 2021;9:631699. doi: 10.3389/fcell.2021.631699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsukiji N, Inoue O, Morimoto M, et al. Platelets play an essential role in murine lung development through Clec-2/podoplanin interaction. Blood. 2018;132(11):1167–1179. doi: 10.1182/blood-2017-12-823369. [DOI] [PubMed] [Google Scholar]
- 61.Bertozzi CC, Schmaier AA, Mericko P, et al. Platelets regulate lymphatic vascular development through CLEC-2–SLP-76 signaling. Blood. 2010;116(4):661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Battinelli EM. In: Platelets. Fourth Edition. Michelson AD, editor. Academic Press; 2019. 24 - The role of platelets in angiogenesis; pp. 433–441. [Google Scholar]
- 63.Tan SYS, Krasnow MA. Developmental origin of lung macrophage diversity. Development. 2016;143(8):1318–1327. doi: 10.1242/dev.129122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakarov S, Lim HY, Tan L, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363(6432) doi: 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- 65.Dick SA, Wong A, Hamidzada H, et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci Immunol. 2022;7(67) doi: 10.1126/sciimmunol.abf7777. [DOI] [PubMed] [Google Scholar]
- 66.Liang G, Zhou C, Jiang X, et al. De novo generation of macrophage from placenta-derived hemogenic endothelium. Dev Cell. 2021;56(14):2121–2133.e6. doi: 10.1016/j.devcel.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Wang H, He J, Xu C, et al. Decoding human megakaryocyte development. Cell Stem Cell. 2021;28(3):535–549.e8. doi: 10.1016/j.stem.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Chen MJ, Li Y, De Obaldia ME, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9(6):541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dignum T, Varnum-Finney B, Srivatsan SR, et al. Multipotent progenitors and hematopoietic stem cells arise independently from hemogenic endothelium in the mouse embryo. Cell Rep. 2021;36(11) doi: 10.1016/j.celrep.2021.109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joseph C, Quach JM, Walkley CR, Lane SW, Lo Celso C, Purton LE. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell. 2013;13(5):520–533. doi: 10.1016/j.stem.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 71.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 72.Dzierzak E, Bigas A. Blood development: hematopoietic stem cell dependence and independence. Cell Stem Cell. 2018;22(5):639–651. doi: 10.1016/j.stem.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 73.Frame JM, McGrath KE, Palis J. Erythro-myeloid progenitors: “definitive” hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells. Blood Cells Mol Dis. 2013;51(4):220–225. doi: 10.1016/j.bcmd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghosn E, Yoshimoto M, Nakauchi H, Weissman IL, Herzenberg LA. Hematopoietic stem cell-independent hematopoiesis and the origins of innate-like B lymphocytes. Development. 2019;146(15) doi: 10.1242/dev.170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soares-da-Silva F, Freyer L, Elsaid R, et al. Yolk sac, but not hematopoietic stem cell–derived progenitors, sustain erythropoiesis throughout murine embryonic life. J Exp Med. 2021;218(4) doi: 10.1084/jem.20201729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busch K, Klapproth K, Barile M, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518(7540):542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- 77.Beaudin AE, Boyer SW, Perez-Cunningham J, et al. A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell. 2016;19(6):768–783. doi: 10.1016/j.stem.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waas B, Maillard I. Fetal hematopoietic stem cells are making waves. Stem Cell Investig. 2017;4:25. doi: 10.21037/sci.2017.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci U S A. 2007;104(22):9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rybtsov S, Sobiesiak M, Taoudi S, et al. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med. 2011;208(6):1305–1315. doi: 10.1084/jem.20102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.