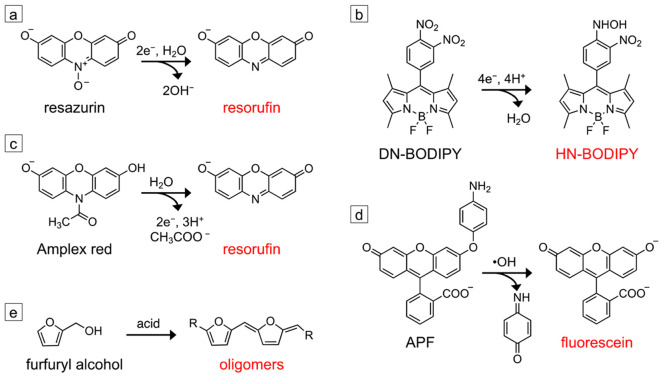
Figure 1.
Fluorogenic probes used for SMF imaging of nanoscale catalysts. (a) Reductive N-deoxygenation of resazurin produces fluorescent resorufin. (b) The reduction of the para-nitro group of 8-(3,4-dinitrophenyl)-1,3,5,7-tetramethyl-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (DN-BODIPY) into a hydroxylamino group produces the fluorescent HN-BODIPY. (c) Oxidative N-deacetylation of Amplex red produces resorufin. (d) Oxidative cleavage of the aminophenyl group of 3′-(p-aminophenyl) fluorescein (APF) produces fluorescein. Both Amplex red and APF can either be directly oxidized by the catalyst to produce the fluorescent product, or they can be activated by reactive oxygen species generated at the catalyst surface. (e) The acid-catalyzed condensation of furfuryl alcohol produces fluorescent oligomers. All probes except the one shown in panel (b) are commercially available. The synthesis of the DN-BODIPY probe is described in ref (187).