Abstract
Introduction
Successful antiviral therapy has transformed HIV infection into a chronic condition, where optimising quality of life (QoL) has become essential for successful lifelong treatment. Patient-reported outcome measures (PROMs) can signal potential physical and mental health problems related to QoL. This study aims to determine whether PROMs in routine clinical care improve quality of care as experienced by people with HIV (PWH).
Methods and analysis
We report the protocol of a multicentre longitudinal cohort studying PWH at Amsterdam University Medical Centres in the Netherlands. PROMs are offered annually to patients via the patient portal of the electronic health record. Domains include anxiety, depression, fatigue, sleep disturbances, social isolation, physical functioning, stigma, post-traumatic stress disorder, adherence, drug and alcohol use and screening questions for sexual health and issues related to finances, housing and migration status. Our intervention comprises (1) patients’ completion of PROMs, (2) discussion of PROMs scores during annual consultations and (3) documentation of follow-up actions in an individualised care plan, if indicated. The primary endpoint will be patient-experienced quality of care, measured by the Patient Assessment of Chronic Illness Care, Short Form (PACIC-S). Patients will provide measurements at baseline, year 1 and year 2. We will explore change over time in PACIC-S and PROMs scores and examine the sociodemographical and HIV-specific characteristics of subgroups of patients who participated in all or only part of the intervention to ascertain whether benefit has been achieved from our intervention in all subgroups.
Ethics and dissemination
Patients provide consent for the analysis of data collected as part of routine clinical care to the AIDS Therapy Evaluation in the Netherlands study (ATHENA) cohort through mechanisms described in Boender et al. Additional ethical approval for the analysis of these data is not required under the ATHENA cohort protocol. The results will be presented at national and international academic meetings and submitted to peer-reviewed journals for publication.
Keywords: Quality of Life, Patient-Reported Outcome Measures, HIV and AIDS, Quality in healthcare, Patient-Centred Care
Strengths and limitations of this study.
This study’s strengths include its multisite, longitudinal design.
Accessing linkages to sociodemographical and HIV-specific data facilitates making inferences about obtained benefit based on patient characteristics.
Our study connects discussing patient-reported outcome measures between patients and healthcare providers in routine clinical care with improvement of patient experience of quality of care.
The absence of a control group is a limitation of this study.
Patients with limited literacy, limited digital literacy and limited access to digital health solutions are potentially the people who might benefit most, but they cannot participate in this study.
Introduction
In the last 40 years, the life expectancy of people living with HIV (PWH) has increased immensely due to the availability of safe and effective antiretroviral treatment transforming the condition into a chronic condition. PWH who enter care without severe HIV-associated complications have a similar life expectancy to those without HIV but lag behind in quality of life (QoL).1 PWH are at greater risk of experiencing multiple chronic comorbidities as they age,2 including cardiovascular diseases, cancers and psychological conditions, such as depression.3 They might also experience stigma and discrimination due to multiple stigmatised identities, including their HIV disease and characteristics that make them vulnerable to HIV, such as their sexuality or migration status.4 Together, increased risk of multiple chronic comorbidities and stigma and discrimination can combine to negatively affect the QoL of PWH.5–7
Patient-reported outcome measures (PROMs) are validated instruments that measure QoL among specific domains, including physical and mental health functioning, stigma, medication adherence, social status, housing, finances and sexuality.8 9 Discussion of PROMs scores between patients and healthcare providers (HCP) as part of routine clinical care for diseases, such as diabetes, arthritis, asthma, cancer and HIV, facilitates shared decision-making9 10; improves communications between patients and HCP9–15; helps to signal potential health problems,15 16 including psychosocial issues11 17 18; and increases patient satisfaction with care.19
For routine clinical care in HIV outpatient clinics, earlier studies have shown that PROMs can help identify previously unnoticed physical and mental health problems,16 20 identify problematic substance use,21 improve adherence15 20 and encourage patient-HCP communication and the development of care plans.17 In our study, we introduce the PWH perspective by exploring whether engagement in PROMs affects patient-experienced quality of care, which can be linked to patient-centredness and system-related chronic care model domains as measured by the Patient Assessment of Chronic Illness Care, Short Form (PACIC-S).22 23
Study aims and hypothesis
The primary objective of our study is to determine whether the quality of routine clinical HIV care as perceived by PWH improves with the introduction of PROMs, which involves patients completing PROMs questionnaires, HCP discussing PROMs scores during annual consultations and documenting follow-up actions in individual care plans, if indicated.
We hypothesise that the experience of quality of care among PWH will improve by introducing PROMs to routine HIV care through the early signalling of physical and psychosocial health problems, followed up with subsequent actions, if indicated.
Methods and analysis
Setting
This is a multicentre intervention studying PWH in care at two of the HIV treatment centres in Amsterdam, the Netherlands, that are affiliated with Amsterdam University Medical Centres (AMC site and VUMC site), together taking care of 2853 individuals. We will limit the analyses to individuals who are part of the ongoing AIDS Therapy Evaluation in the Netherlands study (ATHENA) cohort in which 98% of individuals in care have provided consent. Pseudonymised data transfer and analysis mechanisms for these individuals are managed by Stichting HIV Monitoring on behalf of ATHENA cohort patients through agreements with all treatment centres in the Netherlands, including the two involved in this study.24 Online supplemental appendix 1 in the supplement provides patient and HCP details per site.
bmjopen-2023-073758supp001.pdf (219.3KB, pdf)
Study procedures
PROMs will be sent to people in care once yearly as an integral component of routine care 1–2 weeks prior to their consultation and can be completed in their electronic patient portal. PROMs scores will be discussed with HCP during the annual control consultation. Physicians and nurses in participating centres work together in fixed pairs, which we consider clusters for this study.
Eligibility
Patients 18 years old and above who can engage with healthcare providers in either English or Dutch and who are registered with the electronic patient portal at Amsterdam UMC will be offered the PROMs to complete before their annual consultations.
Recruitment
We will approach consecutive patients in two groups. Group 1 will comprise individuals whose annual control consultations take place in the first 6 months after the rollout of PROMs in the clinics. Rollout will take place sequentially per site. Group 2 will comprise individuals who were approached but who did not complete PROMs in year 1. Group 2 will be offered PROMs once again in year 2 and followed as a separate group.
PROMs selected for routine clinical care
We consulted internal and external stakeholders in late 2020 to determine which domains were most relevant to address the QoL of PWH. Internally, the core team comprising key HIV nurses, infectious disease physicians, a psychiatrist, a social worker and a medical psychologist first assessed the needs of their patient populations and translated these into QoL domains for which PROMs could be implemented. Externally, these were reviewed and adapted by representatives from community organisations, including the national association of PWH (Hiv vereniging), an organisation that works with people who use drugs (Mainline), and by a lawyer specialised in migration law. Members of the PROMs Expertise Centre of Amsterdam UMC provided technical support on the PROMs that would address those domains and will provide training to HCP.
PROMs domains include anxiety, depression, fatigue, sleep disturbances, social isolation, physical functioning, stigma, post-traumatic stress disorder, adherence, drug and alcohol use and screening questions for sexual health and issues related to finances, housing and migration status. Online supplemental appendix 2 in the supplement provides the full list of PROMs selected, their characteristics and their sources. Where possible, we selected Patient-Reported Outcomes Measurement Information System (PROMIS) Computer Adaptive Tests (CATs) for which the selection of items is tailored to the individual based on responses to prior items.25 This minimises the burden on the patient while providing maximally useful information and accuracy.26 Online supplemental appendix 3 provides technical details of which PROMIS instruments were used, and online supplemental appendices 4-8 provide details about the questionnaires that we created or adapted.
Individualised care plan
Individual care plans will be completed by the HCP after the PROMs scores have been discussed at the outpatient clinic. The individual care plans will indicate whether the PROMs have been discussed and describe types of information provided and/or referrals made to other departments within the hospital, medical or allied medical services outside the hospital or community/peer support. Follow-up will take place at the next six monthly consultations unless otherwise agreed on in the consultation.
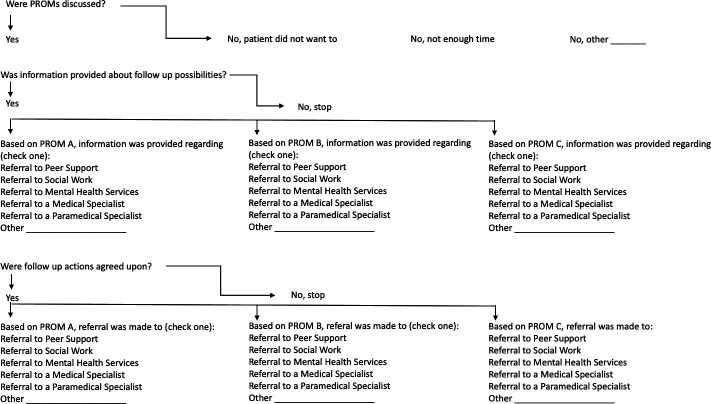
Documentation of the individualised care plan will take place via an electronic form integrated in the electronic health record at AMC and VUMC that leads the HCP through a set of questions related to their clinical findings. Figure 1 shows the logic flow that the template takes to guide the HCP in documenting the individualised care plan. The HCP can document actions for up to three different PROMs, labelled in figure 1 as PROM A, PROM B and PROM C, that represent QoL categories triggered by PROMs scores.
Figure 1.
Individualised care plan flowchart.
Endpoints
The primary endpoint will be patient-experienced quality of care as measured by the PACIC-S, which measures patients’ experiences with how closely services follow the chronic care model.22 23 PACIC-S scores indicating a higher experienced quality of care have been shown to be correlated with PROMs scores indicating a better QoL.23 27 28 This questionnaire is delivered to patients as part of the basic package of PROMs (see online supplemental appendix 2). All other PROMs are secondary outcome measures.
Sample size
To be able to detect a change in our primary outcome, the PACIC-S total score, with an effect size of 0.2 (Cohen’s d, small-sized effect)29 from baseline to the follow-up measurements with 80% power and a two-sided p value of 0.05, a total of 199 patients would be required.
To account for the clustered nature of the data (patients are nested within fixed pairs of HCPs), we will multiply this sample size by a correction factor of 1+(m−1) ρ, where m is the mean expected cluster size and ρ is the anticipated intracluster correlation coefficient.30 We assume an intracluster correlation of ρ=0.017.30 Assuming we will recruit m of 13 patients per cluster, the correction factor is 1.204 for the cluster design. To account for the clustered design, the study would require a total of 240 patients, which we will obtain by approaching consecutive patients until we reach or surpass this number.
Data collection and assessment
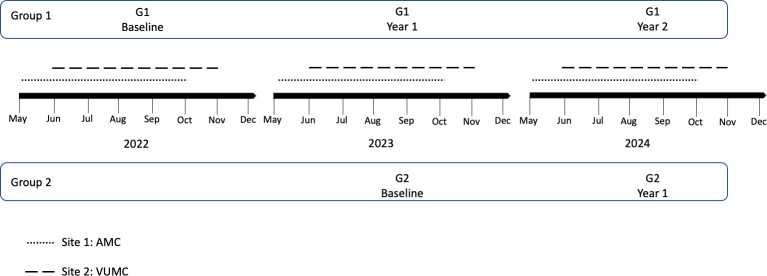
Figure 2 provides the schema for data collection. Group 1 will provide three measurement moments: G1 baseline, G1 year 1 and G1 year 2. Group 2 will provide two measurement moments: G2 baseline and G2 year 1.
Figure 2.
Study timeline and data collection points.
Analysis and statistical considerations
Descriptive statistics
Descriptive data will include PROMs scores; demographics, age, sex, gender, location of treatment centre and country/region of origin; and HIV-specific characteristics, year of diagnosis, viral load suppressed or not and CD4 count.
Statistical analysis
We will compare demographical and HIV-specific characteristics among patients who complete the PROMs, those who received the PROMs and do not complete them and those who were not offered PROMs because they do not have access to the electronic patient portals. We will determine whether our sample is representative of the total patient population using χ2 test and Student’s t-test, analysis of variance (ANOVA) or their non-parametric counterparts were appropriate.
We will analyse changes in the PACIC-S and PROMs scores over time using mixed linear models. The PACIC-S and the PROMs are the dependent variables. Time will be included as a categorical fixed factor (baseline, year 1, year 2). Repeated measurements will be nested within participants to account for the clustering of data within participants. We will include a random intercept on the HCP pairs level to account for the clustering of data within HCP pairs.
We will investigate change over time in PACIC-S and PROMs scores among all patients who were offered the intervention (intention-to-treat population). Additionally, we will explore change over time in PACIC-S and PROMs scores among subgroups of patients (1) with whom PROMs scores were discussed without further follow-up actions, (2) with whom PROMs scores were discussed with subsequent documentation of follow-up activities within individualised care plans and (3) with those who completed the PROMs but where the scores were not discussed with the HCP.
To identify sociodemographical/HIV-specific characteristics significantly associated with obtaining more or less benefit from PROMs, we will conduct series of mixed linear models in which sociodemographical/HIV-specific characteristics will be added one by one as fixed factors to the model that also includes time as fixed factor. The PACIC-S and other PROMs scores will be the dependent variable. Sociodemographical/HIV-specific characteristics with a Wald χ2 test p value <0.20 will be included in further multivariate modelling. Subsequently, sociodemographical/HIV-specific characteristics with p values >0.05 will be removed from the multivariate model using backward elimination.
Two-sided p values <0.05 are considered to indicate statistical significance. Data analysis will be conducted using SPSS V.26 and/or Stata V.16.
Patient and public involvement
The PROMs for routine clinical care were selected with input from the Dutch national HIV patient association. Patients will be involved in piloting the clinical protocol and in the cocreation of tools to support PROMs health literacy, which should lead to increased patient satisfaction.31
Supplementary Material
Footnotes
Contributors: KM, PTN, MB, JFN, AW, KCES, SEG and MVdV contributed to the conception of the study. MVdV is the study chief investigator. PTN performed the power analysis. KM and PTN prepared the first draft of the manuscript for publication. KM is responsible for the study management, with oversight by MVdV, SEG, PTN and MB. KM, PTN, MB, JFN, AW, KCES, LL, CB, HvO, LH, SEG and MVdV contributed to revising the manuscript and approved the final version to be published.
Funding: This work has been supported in part by unrestricted grants from Gilead Sciences and ViiV Healthcare.
Competing interests: KM has received fees for educational activities from Springer Media and Gilead Sciences. JFN has received fees for educational activities from Virology Education, Gilead, ViiV Healthcare, MSD and Astra Zeneca and fees for participation in scientific advisory boards from Gilead Sciences, ViiV Healthcare, MSD and Astra Zeneca all paid to her institution. MvdV has received unrestricted research funding from ViiV, Gilead and fees for participation in scientific advisory boards from Viiv, Gilead Sciences and MSD all paid to his institution. PTN, MB, AW, KCES, LL, CB, HvO, LH and SEG report no competing interests.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.van Sighem AI, Gras LAJ, Reiss P, et al. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 2010;24:1527–35. 10.1097/QAD.0b013e32833a3946 [DOI] [PubMed] [Google Scholar]
- 2.Van Sighem AI, Wit F, Boyd A, et al. Monitoring report 2021. human immunodeficiency virus (HIV) infection in the Netherlands. In: Stichting HIV Monitoring. 2021. Available: https://www.hiv-monitoring.nl/application/files/1816/6851/5357/NL_HIV_MONITORING_REPORT_2022.pdf [accessed 20 Feb 2023]. [Google Scholar]
- 3.Nanni MG, Caruso R, Mitchell AJ, et al. Depression in HIV infected patients: a review. Curr Psychiatry Rep 2015;17:530. 10.1007/s11920-014-0530-4 [DOI] [PubMed] [Google Scholar]
- 4.Nyblade L, Mingkwan P, Stockton MA. Stigma reduction: an essential ingredient to ending AIDS by 2030. Lancet HIV 2021;8:e106–13. 10.1016/S2352-3018(20)30309-X [DOI] [PubMed] [Google Scholar]
- 5.Turan JM, Elafros MA, Logie CH, et al. Challenges and opportunities in examining and addressing Intersectional stigma and health. BMC Med 2019;17:7. 10.1186/s12916-018-1246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logie CH, Wang Y, Lacombe-Duncan A, et al. HIV-related stigma, racial discrimination, and gender discrimination: pathways to physical and mental health-related quality of life among a national cohort of women living with HIV. Prev Med 2018;107:36–44. 10.1016/j.ypmed.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 7.Engelhard EAN, Smit C, van Dijk PR, et al. Health-related quality of life of people with HIV: an assessment of patient related factors and comparison with other chronic diseases. AIDS 2018;32:103–12. 10.1097/QAD.0000000000001672 [DOI] [PubMed] [Google Scholar]
- 8.Kall M, Marcellin F, Harding R, et al. Patient-reported outcomes to enhance person-centred HIV care. Lancet HIV 2020;7:e59–68. 10.1016/S2352-3018(19)30345-5 [DOI] [PubMed] [Google Scholar]
- 9.Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Aff (Millwood) 2016;35:575–82. 10.1377/hlthaff.2015.1362 [DOI] [PubMed] [Google Scholar]
- 10.Bouazza YB, Chiairi I, El Kharbouchi O, et al. Patient-reported outcome measures (Proms) in the management of lung cancer: a systematic review. Lung Cancer 2017;113:140–51. 10.1016/j.lungcan.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 11.Greenhalgh J, Gooding K, Gibbons E, et al. How do patient reported outcome measures (Proms) support clinician-patient communication and patient care? A realist synthesis. J Patient Rep Outcomes 2018;2:42. 10.1186/s41687-018-0061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Detmar SB, Muller MJ, Schornagel JH, et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 2002;288:3027–34. 10.1001/jama.288.23.3027 [DOI] [PubMed] [Google Scholar]
- 13.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well- being: a randomized controlled trial. J Clin Oncol 2004;22:714–24. 10.1200/JCO.2004.06.078 [DOI] [PubMed] [Google Scholar]
- 14.Gibbons C, Porter I, Gonçalves-Bradley DC, et al. Routine provision of feedback from patient-reported outcome measurements to healthcare providers and patients in clinical practice. Cochrane Database Syst Rev 2021;10:CD011589. 10.1002/14651858.CD011589.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Short D, Fredericksen RJ, Crane HM, et al. Utility and impact of the implementation of same-day, self-administered electronic patient-reported outcomes assessments in routine HIV care in two North American clinics. AIDS Behav 2022;26:2409–24. 10.1007/s10461-022-03585-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjær A, Rasmussen TA, Hjollund NH, et al. Patient-reported outcomes in daily clinical practise in HIV outpatient care. Int J Infect Dis 2018;69:108–14. 10.1016/j.ijid.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 17.Jabour SM, Chander G, Riekert KA, et al. The patient reported outcomes as a clinical tool (PROACT) pilot study: what can be gained by sharing computerized patient-reported mental health and substance use symptoms with providers in HIV care AIDS Behav 2021;25:2963–72. 10.1007/s10461-021-03175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valderas JM, Kotzeva A, Espallargues M, et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res 2008;17:179–93. 10.1007/s11136-007-9295-0 [DOI] [PubMed] [Google Scholar]
- 19.Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32:1480–501. 10.1200/JCO.2013.53.5948 [DOI] [PubMed] [Google Scholar]
- 20.Crane HM, Lober W, Webster E, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Curr HIV Res 2007;5:109–18. 10.2174/157016207779316369 [DOI] [PubMed] [Google Scholar]
- 21.Crane HM, Crane PK, Tufano JT, et al. HIV provider documentation and actions following patient reports of at-risk behaviors and conditions when identified by a web-based point-of-care assessment AIDS Behav. AIDS Behav 2017;21:3111–21. 10.1007/s10461-017-1718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cramm JM, Nieboer AP. Factorial validation of the patient assessment of chronic illness care (PACIC) and PACIC short version (PACIC-S) among cardiovascular disease patients in the Netherlands. Health Qual Life Outcomes 2012;10:104. 10.1186/1477-7525-10-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmittdiel J, Mosen DM, Glasgow RE, et al. Patient assessment of chronic illness care (PACIC) and improved patient-centered outcomes for chronic conditions. J Gen Intern Med 2008;23:77–80. 10.1007/s11606-007-0452-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boender TS, Smit C, Sighem A van, et al. AIDS therapy evaluation in the Netherlands (ATHENA) national observational HIV cohort: cohort profile. BMJ Open 2018;8:e022516. 10.1136/bmjopen-2018-022516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cella D, Gershon R, Lai JS, et al. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res 2007;16 Suppl 1:133–41. 10.1007/s11136-007-9204-6 [DOI] [PubMed] [Google Scholar]
- 26.Segawa E, Schalet B, Cella D. A comparison of computer adaptive tests (cats) and short forms in terms of accuracy and number of items administrated using PROMIS profile. Qual Life Res 2020;29:213–21. 10.1007/s11136-019-02312-8 [DOI] [PubMed] [Google Scholar]
- 27.Desmedt M, Vertriest S, Petrovic M, et al. Seen through the patients’ eyes: quality of chronic illness care. Fam Pract 2018;35:446–51. 10.1093/fampra/cmx123 [DOI] [PubMed] [Google Scholar]
- 28.Randell RL, Long MD, Martin CF, et al. Patient perception of chronic illness care in a large inflammatory bowel disease cohort. Inflammatory Bowel Diseases 2013;19:1428–33. 10.1097/MIB.0b013e3182813434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioural sciences. Mahwah, NJ: Erlbaum, 1988. [Google Scholar]
- 30.Killip S, Mahfoud Z, Pearce K. What is an Intracluster correlation coefficient? crucial concepts for primary care researchers. Ann Fam Med 2004;2:204–8. 10.1370/afm.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim S, Morris H, Pizzirani B, et al. Evaluating hospital tools and services that were co-produced with patients: a rapid review. Int J Qual Health Care 2020;32:231–9. 10.1093/intqhc/mzaa020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-073758supp001.pdf (219.3KB, pdf)