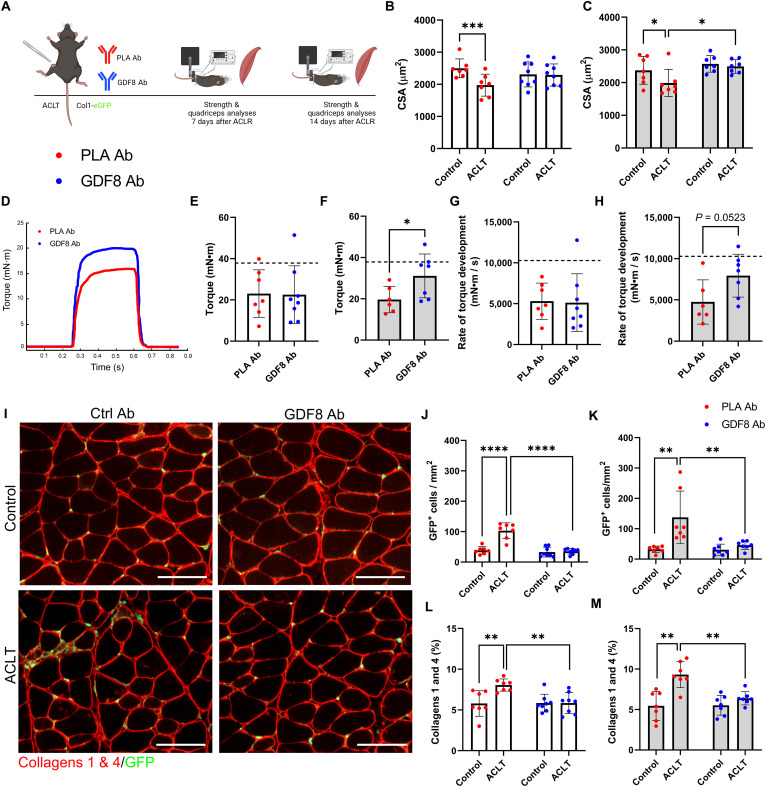
Fig. 2. Humanized monoclonal antibody targeting GDF8 mitigates quadriceps atrophy and weakness and improves muscle quality following ACL injury.
(A) Study diagram; mice were treated biweekly. (B) PLA Ab–treated mice show quadriceps atrophy 1-week after ACL transection (ACLT) (drug × injury interaction, P = 0.002). (C) GDF8 Ab treatment rescues quadriceps fiber atrophy at 2 weeks after ACLT (drug × injury interaction, P = 0.024). (D) Representative tetanic graph from quadriceps peak isometric torque. (E) PLA Ab– and GDF8 Ab–treated mice show similar knee extension torque at 1 week after ACLT. (F) Knee extension weakness is mitigated by GDF8 Ab treatment 2 weeks after ACLT. Dashed lines in (E) and (F) represent mean values from uninjured control mice from historical laboratory data. (G) PLA Ab– and GDF8 Ab–treated mice show similar knee extension rates of torque development at 1 week after ACLT. (H) The knee extension rate of torque development is enhanced by GDF8 Ab treatment 2 weeks after ACLT injury. Dashed lines in (G) and (H) represent mean values from uninjured control mice from historical laboratory data. (I) Representative IHC images of quadriceps collagens 1 and 4 and collagen 1 (Col1)–GFP+ cells. scale bars, 100 μm. (J and K) GDF8 Ab treatment attenuates elevated abundance of Col1-GFP+ cells in quadriceps (J) 1 and (K) 2 weeks after ACLT [drug × injury interaction, P < 0.001 (J) and P = 0.019 (K)]. (L and M) GDF8 Ab treatment blocks the increase in collagens 1 and 4 in quadriceps (L) 1 and (M) 2 weeks after ACLT [drug × injury interaction, P = 0.006 (L) and P = 0.036 (M)]. N = 7 to 8 mice per group. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001 represent Šidák’s multiple comparison post hoc tests performed when significant interactions were detected via mixed models (B, C, J, K, L, and M) or independent sample t test (E to H). PLA Ab, placebo antibody; GDF8 Ab, GDF8 antibody; GFP, green fluorescent protein.