Abstract
Cancer is a major threat to human health and causes death worldwide. Research on the role of radiotherapy (RT) in the treatment of cancer is progressing; however, RT not only causes fatal DNA damage to tumor cells, but also affects the interactions between tumor cells and different components of the tumor microenvironment (TME), including immune cells, fibroblasts, macrophages, extracellular matrix, and some soluble products. Some cancer cells can survive radiation and have shown strong resistance to radiation through interaction with the TME. Currently, the complex relationships between the tumor cells and cellular components that play major roles in various TMEs are poorly understood. This review explores the relationship between RT and cell–cell communication in the TME from the perspective of immunity and hypoxia and aims to identify new RT biomarkers and treatment methods in lung cancer to improve the current status of unstable RT effect and provide a theoretical basis for further lung cancer RT sensitization research in the future.
Keywords: Radiotherapy, Tumor microenvironment, Immune regulation, Hypoxia
Introduction
As of early 2022, cancer remains one of the “major killers” of humans. In recent years, several strategies have been developed to improve cancer treatment.[1] With the continuous improvement in therapies such as neoadjuvant chemotherapy, radiotherapy (RT), targeted therapy, and immunotherapy, cancer mortality has continued to decrease since 1991.[2]
Radiation exposure in tumor cells induces activation of cytoplasmic signaling cascades, such as the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT), RAS/mitogen-activated protein kinase/nuclear factor-κB (NF-κB), signal transducers and transcriptional activators (STAT), and phospholipase C pathways. Radiation-induced activation of these signaling cascades accelerates cell proliferation and tumor cell survival after radiation.[3,4] In addition to the biological functions of cellular regulation described above, activation of these pathways stimulates radiation-induced double-strand break (DSB) repair by modulating or activating the expression of typical DSB repair pathways.[5] Radiation therapy not only kills cancer cells but also triggers the release of proinflammatory mediators and increases the number of tumor-infiltrating immune cells, thus achieving the “cold” to “hot” transformation from an immunological perspective.[6]
Ionizing radiation (IR) not only mediates DNA damage that leads to cancer cell death but also controls tumor progression by influencing the immune system. Its effects on the immune system have two outcomes: immune stimulation and immune suppression. In particular, the immune system plays a crucial role in modulating tumor progression and response to therapy. In this review, we summarized the effects of the tumor microenvironment (TME) on RT-responsive cells, as well as the signaling pathways triggered by TME in tumor cells after receiving RT, leading to reduced RT sensitivity. Targeting these signaling pathways can inhibit tumor growth, induce cancer cell death, overcome tumor resistance to RT, and provide new ideas for further research on tumor RT.
Radiation Promotes Tumor Development Through Immune Cells
The TME, as the name implies, is the internal and external environments in which tumor cells develop, grow, and metastasize. The ongoing interaction between tumor cells and the TME is a bidirectional, dynamic process that plays a decisive role in tumor initiation, progression, metastasis, and response to treatment. The TME typically comprises a large collection of tumor cells, endothelial cells, fibroblasts, neurons, and immune cells, as well as their non-cellular components, including extracellular matrix molecules and soluble products.[7–9] The effect of IR on the TME through its effect on immune cells is one of the main mechanisms by which IR affects tumor occurrence and development.
Tumor-associated Antigen-mediated Radiation to Kill Tumor Cells
Human leukocyte antigen (HLA) is a gene complex encoding major histocompatibility complexes (MHCs), with basic immunomodulatory functions.[10] The MHC plays a key role in acquired immune regulation, recognizing foreign molecules, and determining histocompatibility. The MHC binds to antigens and displays them on the cell surface, activating the corresponding T cells.[11] The MHCs are mainly divided into two types: MHC I and MHC II. Although MHC I and MHC II are relatively similar, there are marked differences in structure and tissue expression patterns. CD8 can recognize MHC I, whereas CD4 can recognize MHC II, which can help T cell receptors (TCR) on the T cell surface specifically recognize MHC-presented peptides.[12,13]
Expression of MHC is generally downregulated in tumor cells, enabling them to evade clearance by the immune system.[14,15] Radiation therapy increases and modulates antigen presentation in cancer cells. Several studies have investigated changes in the appearance of tumor cell surface antigens after radiation. IR upregulates and enriches the expression of MHC-I peptide profile on the tumor cell surface through radiation-induced release of interferon (IFN) type 1 and enhances the lethality of cytotoxic CD8+ T cells to tumor cells.[16]
Some MHC I molecules are upregulated in tumor cells and downregulated after radiation, such as HLA-G and HLA-E, which can promote apoptosis of immune cells,[17,18] downregulate the expression of chemokine receptors on the surface of immune effector cells, and promote tumor escape.[19] These results provide a new approach for the combination of immunotherapy and RT, suggesting that HLA is a promising biomarker and target for tumor imaging and radiosensitization.
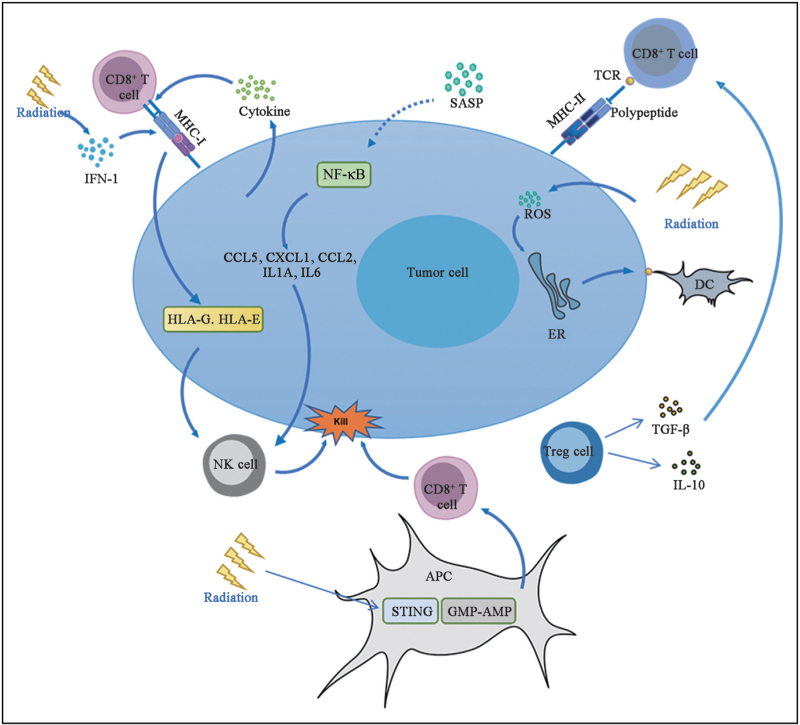
IR damages DNA and causes oxidative stress, leading to cell aging. Several studies have shown that RT can induce cell senescence, both in vitro and in vivo. IR-treated cells presented senescence markers, including P16, P21, and senescence-related secretory phenotypes (SASPs).[20] Cancer-free mice irradiated with sublethal doses of gamma rays also show signs of aging in various tissues. The SASP can stimulate the immune surveillance mechanism and enhance the tumor suppressive function of senescent cells by inducing the immune system to produce an anticancer response. Senescent cells secrete SASP to activate the NF-κB pathway and induce the secretion of cytokines, such as chemokine (C-C motif) ligand (CCL) 5, chemokine (C-X-C motif) ligand 1 (CXCL1), CCL2, transforming growth factor β (TGF β), interleukin (IL)-1A, and IL-6. These cytokines attract macrophages, natural killer (NK) cells, and T cells to mediate immune clearance and cause stagnation of tumor cell growth, thus leading to tumor regression [Figure 1].[21]
Figure 1.
Immunostimulant effect of RT. AMP: Adenosine monophosphate; APC: Antigen presenting cell; CCL2: Chemokine (C-C motif) ligand 2; CCL5: Chemokine (C-C motif) ligand 5; CXCL1: Chemokine (C-X-C motif) ligand 1; DC: Dendritic cell; ER: Endoplasmic reticulum; GMP: Guanosine monophosphate; HLA-E: Human leukocyte antigen E; HLA-G: Human leukocyte antigen G; IFN-1: Type I interferon; IL: Interleukin; MHC-I: Major histocompatibility complex-I; MHC-II: Major histocompatibility complex-II; NF-κB: Nuclear factor-κB; NK: Natural killer; ROS: Reactive oxygen species; RT: Radiotherapy; SASP: Senescence-associated secretory phenotype; STING: Stimulator of interferon genes; TCR: T cell receptor; TGF-β: Transforming growth factor-β; Tregs: Regulatory T cells.
Several challenges are associated with the generation of senescent cells induced by RT. First, SASP promotes mitosis, angiogenesis, and interstitial recombination in residual tumor tissues after RT. It promotes cell survival and proliferation, increases cell stemness, and creates an immunosuppressive environment, leading to tumor recurrence.[22] Second, because most anticancer therapies are administered through systemic approaches, many senescent cells are generated in non-tumor areas, leading to cytotoxicity.[23] This is because of the complexity of the SASP and the non-specificity of the stimulus. Future studies should describe the variables that affect the response to RT by selecting the group of patients most likely to respond favorably to treatment.
IR drives the antitumor immune response through several mechanisms, including the induction of immunogenic cell death (ICD).[24] ICD is a form of stress-induced regulatory cell death that produces antitumor immunity through the release or exposure of damage-associated molecular patterns (DAMPs), which mainly include high-mobility group protein 1 (HMGB1), calreticulin, adenosine triphosphate, and heat shock proteins.[25]
Irradiation of tumor cells results in the production of reactive oxygen species (ROS) and endoplasmic reticulum stress, which are required for DAMP release or exposure.[26,27] After irradiation, calreticulin is translocated, transferred from the perinuclear endoplasmic reticulum to the surface of endoplasmic reticulum, and exposed to the membrane surface, releasing the “eat-me” signal, promoting dendritic cells (DCs) or their progenitor cells to phagocytose dead or dying tumor cells, thus providing rich antigenic substances to promote DCs to mature and perform their functions.[28] When ICD occurs, HMGB1 is released from the cells. The release of HMGB1 acts as a proinflammatory mediator, stimulating monocytes to produce cytokines tumor necrosis factor (TNF), IL-1, IL-6, and IL-8.[25] The released HMGB1 can bind toll-like receptor 4 expressed on the cell surface,[29] activate DCs to phagocytose tumor cells, process tumor antigens, express these tumor antigens and MHC-I molecules on the plasma membrane, and further activate corresponding T cells to achieve immune clearance of tumors.[20] Radiation-induced ICD releases intracellular adenosine triphosphate out of the cell, releasing “find-me” signals to the outside world, which can recruit and promote DC maturation and antigen presentation.[28]
Radiation Stimulates T cells to Kill Cancer Cells
Multiple studies have shown that the presence of tumor-infiltrating lymphocytes, especially effector T cells, before treatment is associated with better survival in cancer patients with different treatment regimens.[30,31]
Radiation therapy increases the diversity of TCR libraries of T cells in tumors. Radiation therapy stimulates the secretion of cytokines necessary for T cell infiltration. Examples include CXC motif chemokines (CXCL9, CXCL10, CXCL11, and CXCL16), which cause T cells to migrate into the TME.[32,33] RT induces tumor cell death, and DNA from dying tumor cells is delivered to antigen-presenting cells (APCs), which activate the guanosine monophosphate-adenosine monophosphate and stimulator of interferon genes pathways in antigen presenting cell, expressing IFN type I, promoting CD8+ T cell infiltration, and increasing tumor cell clearance.[34] In addition, radiation-induced secretion of DAMP-related molecules can also promote the infiltration and maturation of T cells and play an antitumor immune role.
The TME contains not only immune-stimulating molecules but also various immunosuppressive factors, including regulatory T cells (Tregs), macrophages, and myeloid-derived suppressor cells (MDSCs), as well as other stromal cells, such as vascular endothelial cells and cancer-related fibroblasts.
Tregs accumulate and secrete cytokines TGF-β and IL-10 in the TME, which inhibit the activation of effector T cells and stimulate the inhibitory function of MDSC.[35,36] In pancreatic ductal adenocarcinoma, inhibition of Tregs enhances the activation of DC and NK cells, which not only promotes T cell effector function within the TME, but also enhances the immune monitoring of activated NK cells against tumor cell metastasis.[37]
The number of Tregs in tumors and immune organs increases under local or total body irradiation.[38] Tregs are intrinsically resistant to radiation; RT can increase the infiltration of functionally inhibitory Tregs, and the proliferation of Tregs is more vigorous than that of other T cell subsets in the TME.[39] In addition, Tregs profoundly modulate the macrophage phenotype and function. For example, the co-culture of human monocytes with Tregs guides monocytes to differentiate into M2 macrophages, which are involved in the regression of inflammation and tissue remodeling.[40] In addition, macrophages co-cultured with Tregs produce high levels of TGF-β[41]
MDSCs can affect T cell activity by secreting factors that affect amino acid metabolism. Increased arginase-1 activity in MDSCs leads to a decrease in L-arginine, which stops T cells at the G0 and G1 phases.[42] Overexpression of indoleamine 2,3-dioxygenase (IDO) in MDSCs can decompose tryptophan, which is necessary for T cell proliferation and ultimately leads to T cell stagnation at G0.[43] With the depletion of L-tryptophan, activation of the IDO-general control non-derepressible-2 kinase pathway can also upregulate the expression of forkhead box P3 (Foxp3) in CD4+ T cells and induce differentiation into Tregs.[44]
MDSCs secrete a series of ROS and nitrogen substances, such as peroxynitrite, which impair T cell function, thereby inducing nitrification of TCR/CD8 molecules, resulting in changes in TCR/MHC peptide recognition.[45] Moreover, nitrogen monoxide (NO) produced by MDSCs inhibits the Janus kinase (JAK) 3/STAT5 signaling pathway, reduces the expression of MHC class II molecules, and ultimately leads to T cell apoptosis.[46] MDSCs produce ROS that increase Fas ligand expression and decrease B-cell lymphoma-2 (BCL-2) expression, leading to apoptosis of activated T cells.[47] Overall, MDSCs, together with other immunosuppressive cells, form an inhibitory network that attenuates cytotoxic effects on tumor cells.
Irradiation Through DC Induces an Immune Response
Therapeutic agents that enhance the delivery of tumor-associated antigens or amplify the activation signal of DCs can promote antitumor immunity. The CD11c+CD8α+ basic leucine zipper transcription factor, ATF-like 3 (BATF3) lineage DCs have been shown to be key to RT efficacy and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) response.[48,49] And the type I interferon receptor 1 deficiency in CD11c+ DCs reversed the effect.[49] Intratumoral injection of retinoic-acid-inducible gene I agonists enhances DC activation, promoting effective activation of antitumor CD8+ T cells and systemic tumor immunity.[50] Damaged DNA and associated DAMPs released by irradiated tumor cells activate the NF-κB pathway,[51] induce the expression of various proinflammatory genes, increase the level of tumor-associated DCs, enhance the ability of DCs to cross-present antigens, and activate T cells to exert antitumor immunity.[52]
However, contrary to the commonly known antigen presentation process, a portion of the attracted APCs may play a somewhat different role. Current studies have shown that irradiated APCs exert immunosuppressive effects by downregulating the cytokine IL-12. In addition, in the TME, cancer-associated fibroblasts (CAFs) release cyclooxygenase 2-dependent prostaglandin E2 and promote further synthesis of IL-23 by DCs to maintain their immunosuppressive activity.[53] Further insights are needed to elucidate the mechanisms underlying the crosstalk between DCs and tumor cells, particularly the communication between DCs and irradiated surviving tumor cells. In summary, after RT, DCs interact with various components of the TME to create an immunosuppressive environment and promote drug resistance in tumors.
Crosstalk Between Tumor Microenvironment and Radiation Resistance
Radiation resistance is mainly related to two aspects: changes in the microenvironment inside and around the tumor, including DNA damage repair, inflammation, and angiogenesis, and growth signaling pathways. The interaction between cancer stem cells (CSCs) and invasive immune cell populations in the TME is a key factor driving tumor progression, promoting tumor immune escape and enabling tumors to acquire therapeutic resistance.[54] Lung CAFs can reduce the expression of antigen-presenting molecules and costimulatory receptors in monocyte-derived DCs and, to some extent, inhibit their antigen-presenting ability and the ability to activate cytotoxic T cell response.[55] CSCs have stronger DNA repair ability than non-CSCs. Certain CSCs can be transformed into dormant CSCs, which share certain characteristics with “dormant” tumor cells, often leading to therapeutic resistance, metastasis, and immune system evasion. Dormant CSCs also appear to activate intracellular ROS scavenging systems, thereby mitigating damage to DNA caused by ROS and resulting in radiation resistance.[56] In the TME of CSCs, mesenchymal stem cells, CAFs, and exosomes from living cells maintain and promote the phenotypic transformation of CSC through the upregulation of cytokines such as IL-6/IL-8.[57]
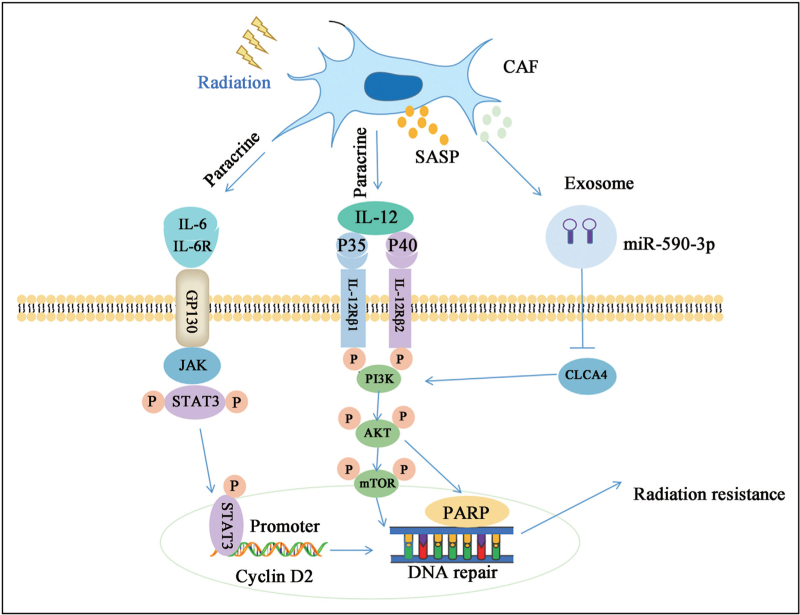
As a rich cell type in the TME, CAFs activation can promote tumor growth, angiogenesis, invasion and metastasis, extracellular matrix remodeling, and chemotherapy resistance in a variety of ways.[56] The CAFs can act as immunosuppressive cells and hypoxic regulators to increase tumor resistance to RT.[57] Immunosuppression plays a key role in regulating cancer progression and metastasis, which is an important factor in determining the efficacy of RT.[58] To grow and survive in the TME, different tumor cells have their own immune escape mechanisms and maintain an immunosuppressant environment to escape the killing effect of immune cells, mainly affecting the recruitment of CD8+ T cells and regulating the activity of CD8+ T cells to escape immune detection.[59] The CAFs are closely related to immune evasion and can activate immunosuppressive cytokines, specifically secreting IL-6 through paracrine signaling to protect cancer cells. Secreted IL-6 activates the JAK1–STAT3 pathway in tumor cells, resulting in chemotherapeutic resistance.[60] In addition, the JAK2/STAT3 signaling pathway plays an important role in the regulation of apoptosis. Activation of this pathway and the phosphorylation of STAT3 leads to the direct binding of STAT3 to the cyclin D2 promoter, which increases the transcription of cyclin D2. This indirectly suggests that CAFs can protect tumor cells from radiation damage through the IL-6/JAK/STAT3/cyclin D2 axis.[61] In addition, IL-22 has been reported as a key mediator for the acquisition of RT resistance in CAFs. Compared with normal fibroblasts, in the cell culture of primary CAFs, IL-22 significantly increased and helped activate the PI3K–AKT–mammalian target of rapamycin (mTOR) signaling pathway to enhance the DNA repair mechanism. It can significantly improve the proliferation, migration, and invasion ability of lung cancer cells, inhibit apoptosis, and reduce the sensitivity of tumor cells to RT [Figure 2].[62,63]
Figure 2.
Molecular mechanism of CAF leading to RT resistance. CAF secretes interleukins and exosomes after radiation stimulation and activates JAK2/STAT3 and PI3K/AKT/mTOR signaling pathways to mediate RT resistance. AKT: Protein kinase B; CAF: Cancer-associated fibroblast; CLCA4: Chloride channel, calcium activated, family member 4; GP130: Glycoprotein 130; IL-6: Interleukin (IL) 6; IL-6R: IL-6 receptor; IL-12: Interleukin 12; IL-12Rβ1: IL-12 receptor β1; IL-12Rβ2: IL-12 receptor β2; JAK: Janus kinase; miR-590-3p: MicroRNA-590-3p; mTOR: Mammalian target of rapamycin; P: Phosphorylation; PARP: Poly adenosine diphosphate (ADP)-ribose polymerase; PI3K: Phosphoinositide 3-kinase; RT: Radiotherapy; SASP: Senescence-associated secretory phenotype; STAT3: Signal transducer and activator of transcription 3.
Extracellular vesicles are membranous vesicles released by cells into the extracellular matrix. They are involved in cell interaction, cell migration, angiogenesis, and tumor cell growth, and are signal carriers that regulate homeostasis during cell development. This cross-linking between cells can be either one- or two-way. Extracellular vesicles can transport biomolecules to recipient cells and maintain long-term stability, making them a natural carrier of drugs and biological agents. Several subtypes of extracellular vesicles have been isolated from mammalian and prokaryotic cell cultures, plasma, and milk, including exosomes, microvesicles, membrane vesicles, and apoptotic bodies.[64–66] Exosome-mediated microRNA delivery by living cells, including cancer cells, contributes to the development of drug resistance in many cancers. CAF-derived exosomes overexpressing miR-590-3p increase cell survival and PI3K/AKT phosphorylation. Through positive regulation of CLCA4-dependent PI3K/AKT signaling pathway, we enhance radioresistance to CRC.[67] It has been reported that miR-24-3p is a key promoter of chemotherapy resistance in various cancers and is transferred to cancer cells by CAF-derived exosomes, downregulates caudal type homeobox transcription factor 2 or Hephaestin expression, inhibits cell apoptosis, and promotes methotrexate resistance.[68] Exosome miR-522 secreted by CAF regulates the expression of arachidonic acid lipoxygenase 15 and reduces the accumulation of lipid ROS in cancer cells, ultimately leading to decreased sensitivity to chemotherapy.[69] Given that intracellular ROS accumulation is an important indicator affecting tumor RT, this could be used as a potential mechanism to improve RT efficiency.[70] In summary, in recent years, an increasing number of studies have been conducted on the involvement of CAF-derived exosomes in regulating the sensitivity of tumor cells to chemoradiotherapy, and an increasing number of mechanisms underlying tumor resistance to chemoradiotherapy have been elaborated.
Tumor-associated macrophages are the most important immune cells in the tumor immune microenvironment. They are usually divided into two distinct functional subtypes, M1 and M2 macrophages. The former exhibits proinflammatory and tumoricidal effects, mediating cytotoxicity and antibody-dependent cell-mediated cytotoxicity to kill tumor cells, whereas the latter exhibits anti-inflammatory and protumor effects, inhibiting T cell-mediated antitumor immune responses.[71] Tumor-associated macrophages secrete inflammatory cytokines and chemokines, such as TNF-α, IL-10, and CCL22, to induce tumor treatment resistance by enhancing CSC and epithelial-mesenchymal transition involvement.[72] In addition, exosomes derived non-coding RNAs from macrophages regulate downstream factors that influence RT sensitivity of tumor cells. Hsa_circ_0001610 was found in exosomes derived from M2 polarized macrophages (EXOs). Hsa_circ_0001610 acts as a competitive endogenous RNA of miR-139-5p in endometrial carcinoma (EC) cells, upregulating cyclin B1 expression and thereby weakening the radiosensitivity of EC cells.[73]
In conclusion, targeting the TME has gradually become the mainstream direction to overcome lung cancer resistance to RT and chemotherapy, suggesting that we need to develop a new strategy for the TME-RT response and improve the survival rate of patients with malignant tumors.
Hypoxic Microenvironment Beneficial to Tumor Cell Resistance to Radiation
Radiation resistance remains a great challenge in tumor treatment, mainly because of tumor desensitization to IR. Tumor cells can consume more glucose and produce more lactic acid than normal cells, even in the presence of normoxic oxygen. These results suggest that reprogramming of glucose metabolism pathways in the TME is an important factor affecting tumor cell proliferation, malignant progression, and chemotherapy and radiation resistance.[74] The hypoxic microenvironment induced by hypoxic signals commonly seen in tumors attenuates the killing effect of IR on tumor cells. Tumor cells are less sensitive to radiation in hypoxic microenvironments than in normal oxygen microenvironments.[75] Hypoxia-inducible factor (HIF) is a transcription factor sensitive to oxygen stress. It consists of an unstable oxygen-sensitive α subunit (HIF-α) and a heterodimer that constitutionally expresses an oxygen-insensitive β subunit (HIF-β). The α subunit includes HIF-1α, HIF-2α, and the less studied HIF-3α.[76] HIF-1α, one of the key regulatory molecules in the HIF-1 signaling pathway, is the most characteristic molecular subtype of HIFs. Canonical regulatory pathways of HIF-1α signaling show that under normoxic conditions, HIF-1α catalyzes the hydroxylation of two proline residues (P402 and P564) in an oxygen-dependent degradation domain in the presence of prolyl hydroxylases in an Fe2+- and α-ketoglutarate-dependent manner. This modification ensures that the oxygen-dependent degradation domain module is degraded by binding to the von Hippel–Lindau tumor suppressor protein, which partially acts as an E3 ubiquitin ligase complex through the ubiquitin–proteasome pathway, leading to HIF-1α polyubiquitin and proteasome degradation, resulting in HIF-1α instability.[76,77]
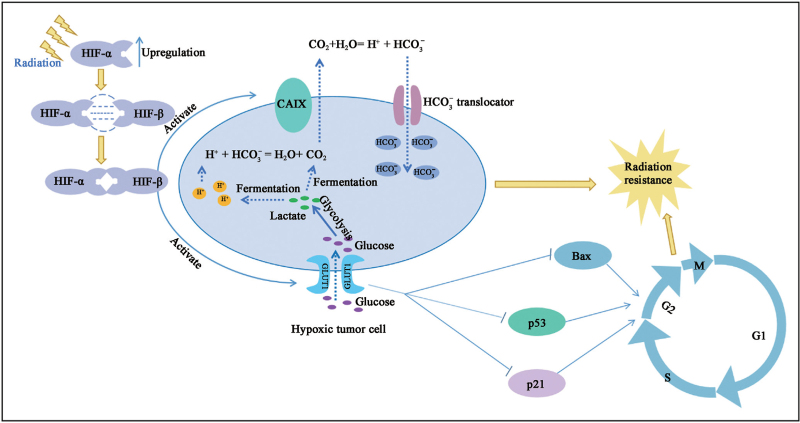
In most solid tumors, rapid tumor growth leads to an excessive oxygen demand, creating an anoxic environment. To adapt to the hypoxic environment, hypoxic tumor cells upregulate the transcription levels of some target genes, which are involved in multiple regulatory processes of tumor cells, such as cell proliferation, reprogramming of cell energy metabolism, apoptosis, and chemotherapy and RT resistance. HIF-1 plays an important role in these processes. HIF-1 upregulation is a predictor of radiation resistance because tumors are highly capable of metastasis and invasion in hypoxic microenvironments.[78] Under hypoxic conditions, HIF-α is amplified in the presence of oxygen-dependent prolyl hydroxylase/factor inhibiting HIF (FIH) inhibition and forms a heterodimer with HIF-β to induce the transcriptional activation of downstream target genes of HIF. These target genes mainly include carbonic anhydrase IX (CAIX), glucose transporter 1 (GLUT1), and vascular endothelial growth factor.[79] CAIX is a hypoxia-induced cell surface glycoprotein that is often expressed in cancer tissues and is used to catalyze the generation of HCO3− and H+ through the action of CO2 and H2O. The generated HCO3− is transported back to tumor cells through the HCO3− transporter and is used to improve intracellular pH. Similarly, CO2 produced by lactic acid fermentation in tumor cells under hypoxic conditions can react with HCO3−, following which extracellular hydration occurs under the catalysis of CAIX to promote its diffusion to the outside of the cell, thus making the extracellular environment more acidic. Because hypoxia and extracellular acidity are considered key factors in radiation resistance of solid tumors, targeting CAIX is also a key direction for radiation-resistant treatment.[80,81] GLUT1 is an important glucose transporter that promotes tumor growth and is responsible for regulating glucose transmembrane transport through the cell membrane, which is a key rate-limiting step in glycolysis. GLUT1 increases the messenger RNA levels of aerobic glycolysis-related genes lactate dehydrogenase A and monocarboxylate transporter 4 and intensifies glucose uptake and lactic acid production [Figure 3]. In addition, GLUT1 plays a role in cell cycle regulation, and its deletion upregulates the levels of the apoptotic genes P53, P21, and BCL-2 associated X protein, but reduces the level of the antiapoptotic gene BCL-2, leading to cell cycle arrest at the G2/M stage, which impairs the radiation resistance of tumor cells.[82,83]
Figure 3.
Intrinsic mechanism of tumor cell radiation resistance caused by hypoxia microenvironment. After receiving radiation stimulation, tumor cells under hypoxia induced enhanced HIF signal, induced activation of downstream target genes CAIX and GLUT1, improved extracellular acidification level, and promoted radiation tolerance of tumor cells. Activated GLUT1 downregulated the levels of apoptotic genes p53, p21 and Bax, promoting G2/M phase transition and consolidating the radiation resistance of tumor cells. Bax: B-cell lymphoma-2 (BCL-2) associated X protein; CAIX: Carbonic anhydrase IX; GLUT1: Glucose transporter 1; HIF: Hypoxia-inducible factor.
Compared with the resistance of HIF-1α to chemotherapy and RT, HIF-2α, which has a structure similar to that of HIF-1, is also involved in the regulation of tumor resistance to RT and chemotherapy. Recent evidence suggests that HIF-2α plays a role in chemotherapy and radiation resistance in solid tumors through mechanisms partially different from those of HIF-1α.[84] High HIF-2α expression leads to radiation resistance in renal cell carcinoma. Lowering HIF-2α levels using mTOR inhibitors can arrest the cell cycle in the G2 phase, as the G2 phase is the main period when IR prevents cell growth.[85,86] Therefore, targeting HIF-2α is beneficial for improving the sensitivity of tumors to RT.
A New Therapeutic Approach: Targeted Therapy and Immunotherapy Combined With Radiotherapy
Treatments that rely on the immune system to kill tumors have made great strides in recent years. In a mouse model with poor immunogenicity of triple-negative breast cancer, RT upregulates the expression of genes containing immunogenicity mutations. Vaccines inoculated with the new epitopes encoded by these genes induce the production of CD8+ and CD4+ T cells, thus effectively improving the therapeutic effect of RT. Furthermore, immunocheckpoint inhibitors such as durvalumab (anti-programmed cell death ligand 1) and ipilimumab (anti-CTLA4) in combination with standard RT significantly extended overall survival in patients with unresected stage III non-small-cell lung cancer.[6,87] These results provide evidence for the synergistic combination of RT and immunotherapy to improve the therapeutic efficacy.
It has long been reported that HIF-1 affects the radiosensitivity of tumors, but the direction and extent of this effect are determined by the TME.[88] Blocking HIF-1 signal can help improve sensitization of RT. Currently, the active HIF-1 inhibitors in the first line of treatment mainly include IDF-11774 and KC7F2, which inhibit the tumor angiogenesis and reduce glucose uptake by reducing the expression of HIF-1 target genes, thus inhibiting the survival and growth of cancer cells under low glucose conditions, and reducing the extracellular acidification rate and oxygen consumption rate of cancer cells.[89–91] In addition, although how HIF-2 influences tumor RT is still unclear, several HIF-2 inhibitors, such as MK-6482 and PT2385, have been reported to inhibit HIF-2 activity by interfering with epigenetic mechanisms, thus inhibiting its downstream effects. The result is that the combination of HIF-2 inhibitors and radiation therapy makes it possible to kill radiation-resistant tumors, and the mechanism remains to be further explored.[92,93]
Conclusion and Perspectives
The TME is a very large aggregate that contains a variety of elements and shows great diversity, which is significantly different from the microenvironment of normal tissues. Different tumor cells can evade the killing effects of radiation through different mechanisms, which are usually related to the interaction between the TME and RT. Therefore, based on the above, it is necessary to explore in-depth the connection between RT and cell–cell communication in the TME, which is very beneficial to future research in the field of RT. With the continuous development of novel RT technologies, design of specific inhibitors targeting CAF and HIF-1/2 is necessary to optimize the therapeutic effect, reduce adverse off-target effects, and overcome RT resistance. The combination of targeted therapy, vascular normalization, immunotherapy, and RT will be a new direction for cancer therapy in the future. How to apply these therapeutic methods into clinical practice remains problematic, for which we still have a difficult task.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82072594 to YT; Nos. 82073097 and 81874139 to SL), Natural Science Foundation of Hunan Province, and Hunan Provincial Key Area Research & Development Programs (No. 2021SK2013 to YT).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang Z, Peng Y, Peng X, Xiao D, Shi Y, Tao Y. Effects of radiation therapy on tumor microenvironment: an updated review. Chin Med J 2023;136:2802–2811. doi: 10.1097/CM9.0000000000002535
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022; 72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun 2020; 40:205–210. doi: 10.1002/cac2.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun M, Li Y, Qian J, Ding S, Sun M, Tan B, et al. Connexin26 modulates the radiosensitivity of cutaneous squamous cell carcinoma by regulating the activation of the MAPK/NF-kappaB signaling pathway. Front Cell Dev Biol 2021; 9:672571.doi: 10.3389/fcell.2021.672571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z, Xu L, Ge Y, Sun H, Zhu J, Dou Q, et al. Cabazitaxel suppresses the proliferation and promotes the apoptosis and radiosensitivity of castration-resistant prostate cancer cells by inhibiting PI3K/AKT pathway. Am J Transl Res 2022; 14:166–181. PMID:35173836. [PMC free article] [PubMed] [Google Scholar]
- 5.Toulany M. Targeting DNA double-strand break repair pathways to improve radiotherapy response. Genes 2019; 10:25.doi: 10.3390/genes10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer 2020; 20:203–217. doi: 10.1038/s41568-020-0246-1. [DOI] [PubMed] [Google Scholar]
- 7.Mohla S. Tumor microenvironment. J Cell Biochem 2007; 101:801–804. doi: 10.1002/jcb.21320. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther 2021; 221:107753.doi: 10.1016/j.pharmthera.2020.107753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci 2008; 13:6537–6553. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- 10.Gallegos CE, Michelin S, Dubner D, Carosella ED. Immunomodulation of classical and non-classical HLA molecules by ionizing radiation. Cell Immunol 2016; 303:16–23. doi: 10.1016/j.cellimm.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol 2006; 18:85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer 2021; 21:298–312. doi: 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- 13.Sivapalan L, Anagnostou V. Genetic variation in antigen presentation and cancer immunotherapy. Immunity 2022; 55:3–6. doi: 10.1016/j.immuni.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibbern ME, Bullock TN, Jenkins TM, Duska LR, Stoler MH, Mills AM. Loss of MHC class I expression in HPV-associated cervical and vulvar neoplasia: a potential mechanism of resistance to checkpoint inhibition. Am J Surg Pathol 2020; 44:1184–1191. doi: 10.1097/PAS.0000000000001506. [DOI] [PubMed] [Google Scholar]
- 15.Smahel M. PD-1/PD-L1 blockade therapy for tumors with downregulated MHC class I expression. Int J Mol Sci 2017; 18:1331.doi: 10.3390/ijms18061331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ugurel S, Uhlig D, Pfohler C, Tilgen W, Schadendorf D, Reinhold U. Down-regulation of HLA class II and costimulatory CD86/B7-2 on circulating monocytes from melanoma patients. Cancer Immunol Immunother 2004; 53:551–559. doi: 10.1007/s00262-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michelin S, Gallegos CE, Dubner D, Favier B, Carosella ED. Ionizing radiation modulates the surface expression of human leukocyte antigen-G in a human melanoma cell line. Hum Immunol 2009; 70:1010–1015. doi: 10.1016/j.humimm.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim EC, Aractingi S, Allory Y, Borrini F, Dupuy A, Duvillard P, et al. Analysis of HLA antigen expression in benign and malignant melanocytic lesions reveals that upregulation of HLA-G expression correlates with malignant transformation, high inflammatory infiltration and HLA-A1 genotype. Int J Cancer 2004; 108:243–250. doi: 10.1002/ijc.11456. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Villa JM, Vaquero-Yuste C, Molina-Alejandre M, Juarez I, Suarez-Trujillo F, Lopez-Nares A, et al. HLA-G: too much or too little? Role in cancer and autoimmune disease. Front Immunol 2022; 13:796054.doi: 10.3389/fimmu.2022.796054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kielbik M, Szulc-Kielbik I, Klink M. Calreticulin - Multifunctional chaperone in immunogenic cell death: potential significance as a prognostic biomarker in ovarian cancer patients. Cells 2021; 10:130.doi: 10.3390/cells10010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao SG, Jackson JG. SASP: tumor suppressor or promoter? Yes! Trends Cancer 2016; 2:676–687. doi: 10.1016/j.trecan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Mavrogonatou E, Pratsinis H, Kletsas D. The role of senescence in cancer development. Semin Cancer Biol 2020; 62:182–191. doi: 10.1016/j.semcancer.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Kohli J, Demaria M. Senescent cells in cancer therapy: friends or foes? Trends Cancer 2020; 6:838–857. doi: 10.1016/j.trecan.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015; 16:e498–e509. doi: 10.1016/s1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 25.Hu ZI, McArthur HL, Ho AY. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr Breast Cancer Rep 2017; 9:45–51. doi: 10.1007/s12609-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radogna F, Diederich M. Stress-induced cellular responses in immunogenic cell death: implications for cancer immunotherapy. Biochem Pharmacol 2018; 153:12–23. doi: 10.1016/j.bcp.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med 2019; 23:4854–4865. doi: 10.1111/jcmm.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev 2017; 280:126–148. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 29.Kashani B, Zandi Z, Pourbagheri-Sigaroodi A, Bashash D, Ghaffari SH. The role of toll-like receptor 4 (TLR4) in cancer progression: a possible therapeutic target? J Cell Physiol 2021; 236:4121–4137. doi: 10.1002/jcp.30166. [DOI] [PubMed] [Google Scholar]
- 30.Characiejus D, Pasukoniene V, Jacobs JJ, Eidukevicius R, Jankevicius F, Dobrovolskiene N, et al. Prognostic significance of peripheral blood CD8highCD57+ lymphocytes in bladder carcinoma patients after intravesical IL-2. Anticancer Res 2011; 31:699–703. [PubMed] [Google Scholar]
- 31.Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Publisher correction: single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 2018; 24:1941.doi: 10.1038/s41591-018-0176-6. [DOI] [PubMed] [Google Scholar]
- 32.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozpiskin OM, Zhang L, Li JJ. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics 2019; 9:1215–1231. doi: 10.7150/thno.32648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darragh LB, Oweida AJ, Karam SD. Overcoming resistance to combination radiation-immunotherapy: a focus on contributing pathways within the tumor microenvironment. Front Immunol 2018; 9:3154.doi: 10.3389/fimmu.2018.03154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discov 2019; 18:749–769. doi: 10.1038/s41573-019-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucca LE, Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat Rev Immunol 2020; 20:680–693. doi: 10.1038/s41577-020-0296-3. [DOI] [PubMed] [Google Scholar]
- 37.Piper M, Van Court B, Mueller A, Watanabe S, Bickett T, Bhatia S, et al. Targeting Treg-expressed STAT3 enhances NK-mediated surveillance of metastasis and improves therapeutic response in pancreatic adenocarcinoma. Clin Cancer Res 2022; 28:1013–1026. doi: 10.1158/1078-0432.CCR-21-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Formenti SC, Demaria S. Future of radiation and immunotherapy. Int J Radiat Oncol Biol Phys 2020; 108:3–5. doi: 10.1016/j.ijrobp.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 39.Muroyama Y, Nirschl TR, Kochel CM, Lopez-Bujanda Z, Theodros D, Mao W, et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol Res 2017; 5:992–1004. doi: 10.1158/2326-6066.CIR-17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A 2007; 104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt A, Zhang XM, Joshi RN, Iqbal S, Wahlund C, Gabrielsson S, et al. Human macrophages induce CD4(+)Foxp3(+) regulatory T cells via binding and re-release of TGF-beta. Immunol Cell Biol 2016; 94:747–762. doi: 10.1038/icb.2016.34. [DOI] [PubMed] [Google Scholar]
- 42.Heuvers ME, Muskens F, Bezemer K, Lambers M, Dingemans AC, Groen HJM, et al. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer 2013; 81:468–474. doi: 10.1016/j.lungcan.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Baumjohann D, Kageyama R, Clingan JM, Morar MM, Patel S, de Kouchkovsky D, et al. The microRNA cluster miR-17 approximately 92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nat Immunol 2013; 14:840–848. doi: 10.1038/ni.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol 2010; 185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 2009; 182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Shen XF, Cao K, Ding J, Kang X, Guan WX, et al. Dexamethasone-induced myeloid-derived suppressor cells prolong allo cardiac graft survival through iNOS- and glucocorticoid receptor-dependent mechanism. Front Immunol 2018; 9:282.doi: 10.3389/fimmu.2018.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MH, Hong SH, Park C, Kim GY, Leem SH, Choi SH, et al. Hwang-Heuk-San induces apoptosis in HCT116 human colorectal cancer cells through the ROS-mediated activation of caspases and the inactivation of the PI3K/Akt signaling pathway. Oncol Rep 2016; 36:205–214. doi: 10.3892/or.2016.4812. [DOI] [PubMed] [Google Scholar]
- 48.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017; 8:15618.doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014; 41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017; 17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 51.Simon PS, Bardhan K, Chen MR, Paschall AV, Lu C, Bollag RJ, et al. NF-(B functions as a molecular link between tumor cells and Th1/Tc1 T cells in the tumor microenvironment to exert radiation-mediated tumor suppression. Oncotarget 2016; 7:23395–23415. doi: 10.18632/oncotarget.8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spiotto M, Fu YX, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol 2016; 1:EAAG1266.doi: 10.1126/sciimmunol.aag1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malecka A, Wang Q, Shah S, Sutavani RV, Spendlove I, Ramage JM, et al. Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation. J Leukoc Biol 2016; 100:381–389. doi: 10.1189/jlb.3A1015-474R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer 2021; 21:526–536. doi: 10.1038/s41568-021-00366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berzaghi R, Tornaas S, Lode K, Hellevik T, Martinez-Zubiaurre I. Ionizing radiation curtails immunosuppressive effects from cancer-associated fibroblasts on dendritic cells. Front Immunol 2021; 12:662594.doi: 10.3389/fimmu.2021.662594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang B, Yan X, Li Y. Cancer stem cell for tumor therapy. Cancers 2021; 13:4814.doi: 10.3390/cancers13194814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taeb S, Ashrafizadeh M, Zarrabi A, Rezapoor S, Musa AE, Farhood B, et al. Role of tumor microenvironment in cancer stem cells resistance to radiotherapy. Curr Cancer Drug Targets 2022; 22:18–30. doi: 10.2174/1568009622666211224154952. [DOI] [PubMed] [Google Scholar]
- 58.Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells 2020; 9:561.doi: 10.3390/cells9030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol 2019; 234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 60.Ham IH, Oh HJ, Jin H, Bae CA, Jeon SM, Choi KS, et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol Cancer 2019; 18:68.doi: 10.1186/s12943-019-0972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park SY, Lee CJ, Choi JH, Kim JH, Kim JW, Kim JY, et al. The JAK2/STAT3/CCND2 axis promotes colorectal cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res 2019; 38:399.doi: 10.1186/s13046-019-1405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marquard FE, Jucker M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem Pharmacol 2020; 172:113729.doi: 10.1016/j.bcp.2019.113729. [DOI] [PubMed] [Google Scholar]
- 63.Li H, Zhang Q, Wu Q, Cui Y, Zhu H, Fang M, et al. Interleukin-22 secreted by cancer-associated fibroblasts regulates the proliferation and metastasis of lung cancer cells via the PI3K-Akt-mTOR signaling pathway. Am J Transl Res 2019; 11:4077–4088. [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma S, Masud MK, Kaneti YV, Rewatkar P, Koradia A, Hossain MSA, et al. Extracellular vesicle nanoarchitectonics for novel drug delivery applications. Small 2021; 17:e2102220.doi: 10.1002/smll.202102220. [DOI] [PubMed] [Google Scholar]
- 65.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol 2021; 16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 66.Murali VP, Holmes CA. Biomaterial-based extracellular vesicle delivery for therapeutic applications. Acta Biomater 2021; 124:88–107. doi: 10.1016/j.actbio.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 67.Chen X, Liu Y, Zhang Q, Liu B, Cheng Y, Zhang Y, et al. Exosomal miR-590-3p derived from cancer-associated fibroblasts confers radioresistance in colorectal cancer. Mol Ther Nucleic Acids 2021; 24:113–126. doi:10.1016/j.omtn.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez de Andres J, Grinan-Lison C, Jimenez G, Marchal JA. Cancer stem cell secretome in the tumor microenvironment: a key point for an effective personalized cancer treatment. J Hematol Oncol 2020; 13:136.doi: 10.1186/s13045-020-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 2020; 19:43.doi: 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ying HJ, Fang M, Chen M. Progress in the mechanism of radiation-induced lung injury. Chin Med J 2020; 134:161–163. doi: 10.1097/CM9.0000000000001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol 2020; 30:R246–R248. doi: 10.1016/j.cub.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 72.Xiao M, He J, Yin L, Chen X, Zu X, Shen Y. Tumor-associated macrophages: critical players in drug resistance of breast cancer. Front Immunol 2021; 12:799428.doi: 10.3389/fimmu.2021.799428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu X, Shi Y, Dong M, Jiang L, Yang J, Liu Z. Exosomal transfer of tumor-associated macrophage-derived hsa_circ_0001610 reduces radiosensitivity in endometrial cancer. Cell Death Dis 2021; 12:818.doi: 10.1038/s41419-021-04087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol 2021; 599:1745–1757. doi: 10.1113/JP278810. [DOI] [PubMed] [Google Scholar]
- 75.Gong L, Zhang Y, Liu C, Zhang M, Han S. Application of radiosensitizers in cancer radiotherapy. Int J Nanomedicine 2021; 16:1083–1102. doi: 10.2147/IJN.S290438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li T, Mao C, Wang X, Shi Y, Tao Y. Epigenetic crosstalk between hypoxia and tumor driven by HIF regulation. J Exp Clin Cancer Res 2020; 39:224.doi: 10.1186/s13046-020-01733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kabakov AE, Yakimova AO. Hypoxia-induced cancer cell responses driving radioresistance of hypoxic tumors: Approaches to targeting and radiosensitizing. Cancers 2021; 13:1102.doi: 10.3390/cancers13051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattarai D, Xu X, Lee K. Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade (2007 to 2016): a “structure-activity relationship” perspective. Med Res Rev 2018; 38:1404–1442. doi: 10.1002/med.21477. [DOI] [PubMed] [Google Scholar]
- 79.Moreno Roig E, Groot AJ, Yaromina A, Hendrickx TC, Barbeau LMO, Giuranno L, et al. HIF-1alpha and HIF-2alpha differently regulate the radiation sensitivity of NSCLC cells. Cells 2019; 8:45.doi: 10.3390/cells8010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ward C, Meehan J, Gray M, Kunkler IH, Langdon SP, Argyle DJ. Carbonic anhydrase IX (CAIX), cancer, and radiation responsiveness. Metabolites 2018; 8:13.doi: 10.3390/metabo8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu P, Zhang Y, Ge F, Zhang F, He X, Gao X. Modulation of tumor microenvironment to enhance radiotherapy efficacy in esophageal squamous cell carcinoma by inhibiting carbonic anhydrase IX. Front Oncol 2021; 11:637252.doi: 10.3389/fonc.2021.637252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao H, Wang J, Yan W, Cui Y, Chen Z, Gao X, et al. GLUT1 regulates cell glycolysis and proliferation in prostate cancer. Prostate 2018; 78:86–94. doi: 10.1002/pros.23448. [DOI] [PubMed] [Google Scholar]
- 83.Yang M, Li H, Rong M, Zhang H, Hou L, Zhang C. Dysregulated GLUT1 may be involved in the pathogenesis of preeclampsia by impairing decidualization. Mol Cell Endocrinol 2022; 540:111509.doi: 10.1016/j.mce.2021.111509. [DOI] [PubMed] [Google Scholar]
- 84.Zhao J, Du F, Luo Y, Shen G, Zheng F, Xu B. The emerging role of hypoxia-inducible factor-2 involved in chemo/radioresistance in solid tumors. Cancer Treat Rev 2015; 41:623–633. doi: 10.1016/j.ctrv.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 85.Anbumani S, Mohankumar MN. Gamma radiation induced cell cycle perturbations and DNA damage in Catla Catla as measured by flow cytometry. Ecotoxicol Environ Saf 2015; 113:18–22. doi: 10.1016/j.ecoenv.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 86.Bhatt RS, Landis DM, Zimmer M, Torregrossa J, Chen S, Sukhatme VP, et al. Hypoxia-inducible factor-2alpha: effect on radiation sensitivity and differential regulation by an mTOR inhibitor. BJU Int 2008; 102:358–363. doi: 10.1111/j.1464-410X.2008.07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lhuillier C, Rudqvist NP, Yamazaki T, Zhang T, Charpentier M, Galluzzi L, et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest 2021; 131:e138740.doi: 10.1172/JCI138740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moeller BJ, Dewhirst MW. HIF-1 and tumour radiosensitivity. Br J Cancer 2006; 95:1–5. doi:10.1038/sj.bjc.6603201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Philip M, Mathew B, Karatt TK, Perwad Z, Subhahar MB, Karakka Kal AK, et al. Metabolic studies of hypoxia-inducible factor stabilisers IOX2, IOX3 and IOX4 (in vitro) for doping control. Drug Test Anal 2021; 13:794–816. doi: 10.1002/dta.3000. [DOI] [PubMed] [Google Scholar]
- 90.Chen C, Yan S, Geng ZD, Wang Z. Fracture repair by IOX2: regulation of the hypoxia inducible factor-1alpha signaling pathway and BMSCs. Eur J Pharmacol 2022; 921:174864.doi:10.1016/j.ejphar.2022.174864. [DOI] [PubMed] [Google Scholar]
- 91.Ban HS, Kim BK, Lee H, Kim HM, Harmalkar D, Nam M, et al. The novel hypoxia-inducible factor-1alpha inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth. Cell Death Dis 2017; 8:e2843.doi: 10.1038/cddis.2017.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasanov E, Jonasch E. MK-6482 as a potential treatment for von Hippel-Lindau disease-associated clear cell renal cell carcinoma. Expert Opin Investig Drugs 2021; 30:495–504. doi:10.1080/13543784.2021.1925248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Courtney KD, Ma Y, Diaz de Leon A, Christie A, Xie Z, Woolford L, et al. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin Cancer Res 2020; 26:793–803. doi:10.1158/1078-0432.CCR-19-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]