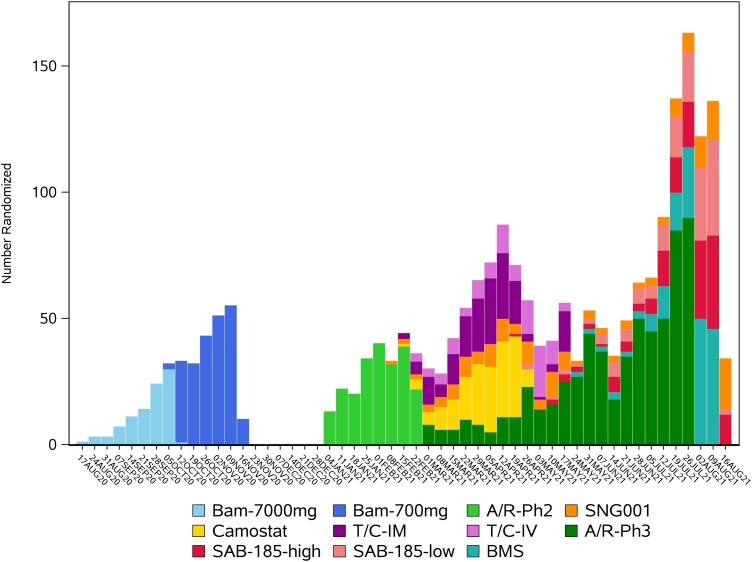
Figure 3.
Enrollment over time for the placebo-controlled evaluation of agents in ACTIV-2. Abbreviations: A/R-Ph2, amubarvimab/romlusevimab phase 2; A/R-Ph3, amubarvimab/romlusevimab phase 3; Bam-700 mg, bamlanivimab 700 mg; Bam-7000 mg, bamlanivimab 7000 mg; BMS, BMS-986414 + BMS-986413; SAB-185-high, SAB-185 high dose; SAB-185-low, SAB-185 low dose; T/C IV, tixagevimab/cilgavimab by intravenous infusion; T/C-IM, tixagevimab/cilgavimab by intramuscular injection.