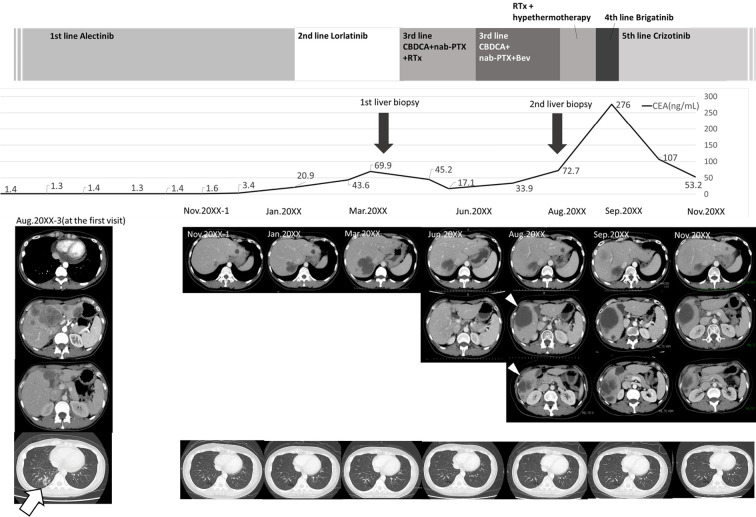
Figure 1.
Clinical course of the patient. The patient was diagnosed with stage IVB ALK-positive lung adenocarcinoma in August 20XX-3 and given alectinib as first-line treatment. A positive treatment effect continued for about 2.5 years. However, in January 20XX, liver metastases rapidly developed, and the patient was switched to lorlatinib as second-line treatment. Nevertheless, the liver metastases increased further, and levels of tumor markers also tended to increase. A liver biopsy was performed to confirm the resistance mechanism, but an adequate specimen could not be obtained. The treatment regimen was changed to third-line CBDCA+nab-PTX in April 20XX, and BEV was started in June 20XX. However, computed tomography in August 20XX revealed new liver metastases (white arrowheads), and a second liver biopsy of these metastases was performed. The biopsied specimens were subjected to next-generation sequencing. In September 20XX, before obtaining the results of the analysis, palliative radiotherapy and hyperthermia therapy were administered, and brigatinib was started as fourth-line treatment. Despite these treatments, growth of the liver metastases and peritoneal dissemination were observed. After the second liver biopsy confirmed MET amplification, crizotinib was administered as fifth-line treatment, and all liver metastases were reduced in size. A marked reduction in the CEA level was also observed. During the entire treatment period, the primary lesion in the lower right lobe (white arrow) did not increase. ALK: anaplastic lymphoma kinase, BEV: bevacizumab, CBDCA: carboplatin, CEA: carcinoembryonic antigen, MET: mesenchymal-epithelial transition gene, nab-PTX: nanoparticle albumin-bound paclitaxel, RTx: radiation therapy