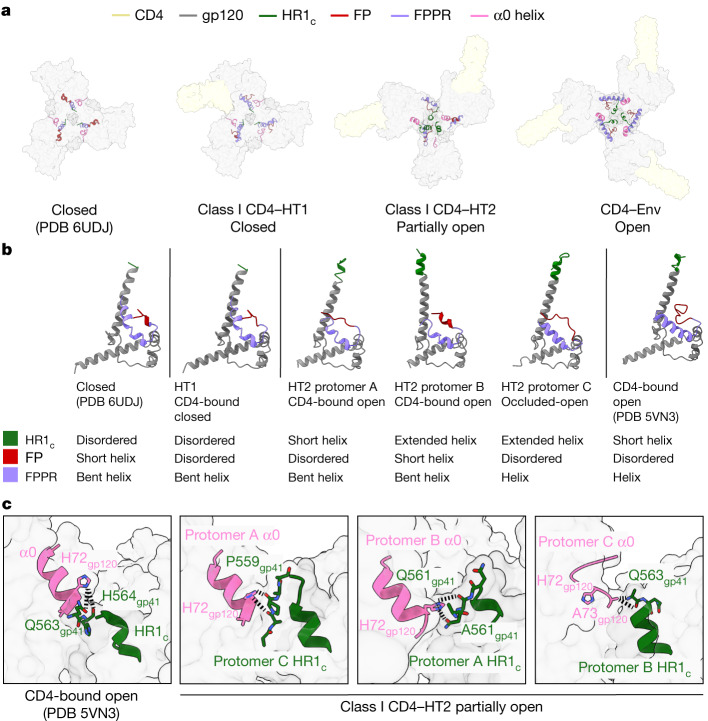
Fig. 3. Conformational changes in gp41 were coordinated with gp120 conformation in CD4-bound heterotrimers.
a, Top-down views of surface representations of Envs from closed (PDB 6UDJ), class I CD4–HT1, class I CD4–HT2 and CD4–Env (PDB 5VN3) structures with gp41 structural elements (HR1c, fusion peptide (FP), FPPR, α0 helix) depicted in cartoon representations. b, Cartoon representations of gp41 subunits from closed (PDB 6UDJ), class I CD4–HT1, class I CD4–HT2 (protomers A–C) and CD4–Env (PDB 5VN3) structures with coloured gp41 (HR1c, FP, FPPR, α0 helix) structural elements. c, Cartoon representations of the α0 helix and HR1c for CD4–Env (PDB 5VN3) and class I CD4–HT2 (protomers A–C) with stick representations of selected amino acids. Black dashed connecting lines indicate gp120–gp41 interactions within 6.0 Å.