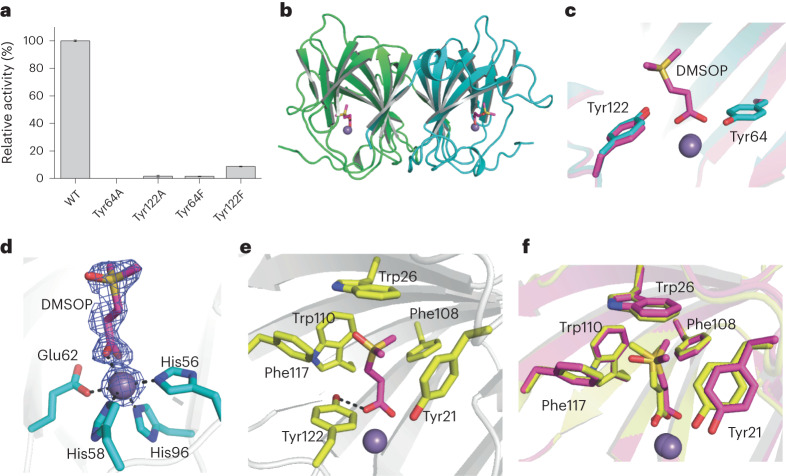
Fig. 5. Structural and mutational analyses of DddK.
a, DMSOP lyase activity of purified site-directed DddK mutant proteins where residues potentially involved in DMSOP catabolism were substituted as indicated. The enzymatic activity of WT DddK was defined as 100%. Results represent the mean of three independent experiments with error bars showing the respective s.d. b, Overall structure of the DddK–DMSOP complex. There are two DddK molecules arranged as a dimer in an asymmetric unit, which are coloured in green and cyan, respectively. The metal ion in DddK is shown as a purple sphere. The DMSOP molecule is shown in magenta sticks. c, Structural alignment of the DddK–DMSOP complex and WT DddK (PDB code: 6A53). The structure of DddK–DMSOP complex is shown in magenta, and the structure of WT DddK complex is shown in cyan. d, Residues and molecules involved in coordinating Mn2+ in DddK. The 2Fo-Fc densities for DMSOP and Mn2+ are contoured in blue meshes at 1.0σ. e, Residues involved in binding DMSOP. f, Structural alignment of important residues from DddK–DMSOP complex and DddK–DMSP complex (PDB code: 6A55). The structure of DddK–DMSOP complex is coloured in magenta, and the structure of DddK–DMSP complex in yellow.