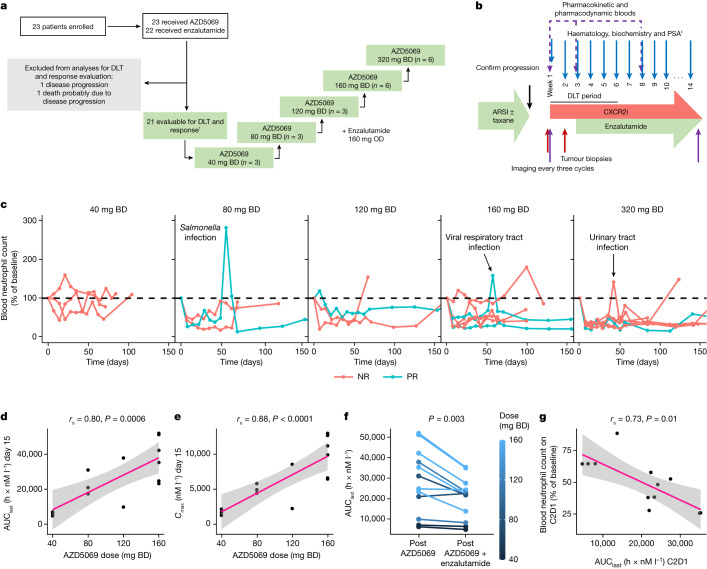
Fig. 2. CXCR2 blockade leads to dose-dependent, on-target neutropaenia.
a, Patient disposition per Consolidated Standards of Reporting Trials guidelines. †Two patients were replaced per protocol after coming off study before completing the DLT period for a reason other than a DLT, and therefore were not evaluable for the primary endpoint or response. b, Clinical trial schema. Patients had confirmed disease progression on androgen deprivation therapy and at least one ARSI. Week count relative to the commencement of AZD5069 administration is shown. Cohorts 1–4 started AZD5069 2 weeks before enzalutamide; cohort 5 started drugs concurrently. ‡PSA test was carried out on day 1 of each cycle. c, By-patient, serial, peripheral blood neutrophil counts for each dose level of AZD5069. All available data points up to day 150 are shown. NR, patient classed as a non-responder; PR, patient classed as a partial responder. d, Scatterplot of AZD5069 dose versus AUClast (h × nM l−1) for AZD5069 monotherapy on day 15 of AZD5069 administration at 40 to 160 mg BD (n = 14). e, Scatterplot of AZD5069 dose and peak concentration (Cmax (nM l−1)) on day 15 of AZD5059 administration in patients treated with AZD5069 at 40 to 160 mg BD (n = 14). f, AZD5069 plasma concentration (AUClast (h × nM l−1)) at steady state for AZD5069 monotherapy (after 14 days of monotherapy) versus combination therapy (after 28 days of combined administration of AZD5069 and enzalutamide; n = 12 pairs). Two-sided paired Wilcoxon signed-rank test P value is shown. Line colour indicates AZD5069 dose. g, Scatterplot of AZD5069 plasma concentration on cycle 2 day 1 (C2D1) (x axis) and blood neutrophil count on C2D1 as a percentage of the value at baseline. For d,e,g, estimated linear regression lines (pink) with 95% confidence interval (grey band), and correlation coefficients and P values from the two-sided Spearman’s rank correlation analyses are shown.