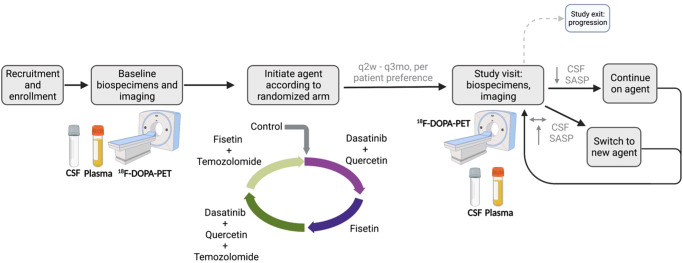
Fig. 2. Candidate clinical trial design for latent glioma.
In patients with latent glioma and CSF access devices, baseline biospecimens and imaging would be acquired prior to initiation of a sequence of senolytics. Biospecimens and imaging would be acquired every 2 weeks to 3 months to assess for pharmacodynamic response based on the CSF SASP. Lack of response would lead to discontinuation of that agent and initiation of the next one in the sequence.