Figure 1.
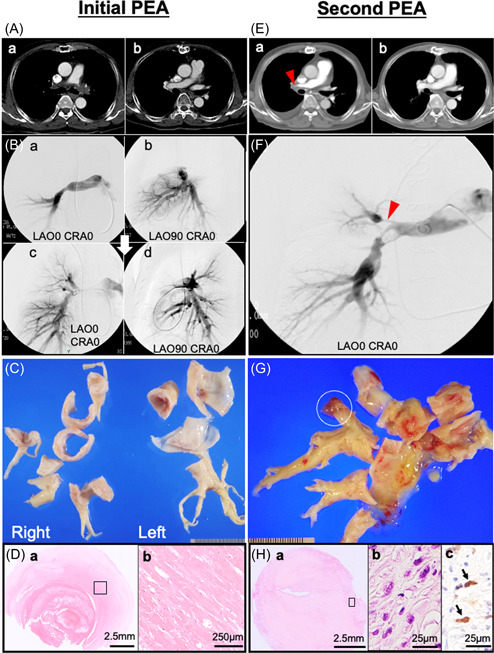
(A‐a) Computed tomography (CT) imaging before the first pulmonary endarterectomy (PEA) showing a filling defect in the main to bilateral pulmonary arteries. (B‐a, b) Pulmonary arteriography showing total occlusion of the left main pulmonary artery (PA) and multiple occlusions and narrowing of the right PA at the lobar level. (A‐b) CT and (B‐c, d) angiography after PEA showing improvement of distal perfusion in both pulmonary arteries. (C) Gross images of specimens obtained during the PEA showing a solid non‐mass‐forming lesion extending from the proximal PA to the sublobar branches of the pulmonary arteries. (D‐a) Microscopic image showing a fresh thrombus attached to the organized thrombus in the background of a sclerosing extracellular matrix (hematoxylin & eosin staining: H&E). (D‐b) High power view of the open square area of D‐a showing no tumor cells (H&E). (E‐a) CT imaging before the second PEA showing a filling defect in the proximal section of the right PA (red arrowhead). (E‐b) Improved contrast enhancement on CT after the second PEA. (F) Angiography showing occlusions and severe stenosis with a thread‐like appearance of the right PA (red arrowhead). (G) The PA mold specimen obtained during the second PEA shows a toffee‐like appearance with a gelatinous mass in a distal tip (white open circle). (H‐a) Microscopically, a myxomatous area containing proliferation of atypical spindle/pleomorphic cells (H&E). (H‐b) High power view of the open square area of H‐a showing atypical spindle cells (H‐b: H&E, H‐c: MDM2 immunohistochemical staining). The nuclei of spindle/pleomorphic cells positive for MDM2 (arrow).
