Abstract
Background/Aim: Circulating tumor DNA (ctDNA), which is shed from cancer cells into the bloodstream, offers a potential minimally invasive approach for cancer diagnosis and monitoring. This research aimed to assess the preoperative ctDNA levels in ovarian tumors patients' plasma and establish correlations with clinicopathological parameters and patient prognosis.
Patients and Methods: Tumor DNA was extracted from ovarian tumor tissue from 41 patients. Targeted sequencing using a panel of 127 genes recurrently mutated in cancer was performed to identify candidate somatic mutations in the tumor DNA. SAGAsafe digital PCR (dPCR) assays targeting the candidate mutations were used to measure ctDNA levels in patient plasma samples, obtained prior to surgery, to evaluate ctDNA levels in terms of mutant copy number/ml and variant allele frequency.
Results: Somatic mutations were found in 24 tumor samples, 17 of which were from ovarian cancer patients. The most frequently mutated gene was TP53. Preoperative plasma ctDNA levels were detected in 14 of the 24 patients. With higher stage, plasma ctDNA mutant concentration increased (p for trend <0.001). The overall survival of cancer patients with more than 10 ctDNA mutant copies/ml in plasma was significantly worse (p=0.008).
Conclusion: Pre-operative ctDNA measurement in ovarian cancer patients' plasma holds promise as a predictive biomarker for tumor staging and prognosis.
Keywords: ctDNA, ovarian cancer, overall survival
Ovarian cancer (OC) is one of the most prevalent and lethal gynecological cancers with more than 300,000 new cases diagnosed and more than 200,000 deaths recorded in 2020 worldwide (1). The 5-year survival rate of ovarian cancer has remained in the range of 40-50% in the past 20 years despite diagnostic and therapeutic advancements (2). When diagnosed early, the prognosis of ovarian cancer is much better, with a 5-year survival rate of 73-92% for tumors diagnosed at stage I, compared to <6% for tumors diagnosed at stage IV (3).
Early detection of minimal disease can be crucial for early surgical intervention and improve the outcome for the patient. However, due to the lack of suitable biomarkers, effective screening of ovarian cancer is yet to be established. Population screening trials like the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) (4) and Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) (5) have been performed to improve screening to enable earlier diagnosis of ovarian cancer; however still no excellent screening method has been found. For women with average to high risk of developing ovarian cancer, transvaginal ultrasound (TVUS) and CA-125 and HE4 blood tests are advised for screening of the disease, but no significant improvement in survival has been observed with these tests (6). Protein biomarkers and combinations of biomarkers have good sensitivity to indicate malignancy in women with pelvic mass, but the specificity needs to be improved when women with pelvic tumors are evaluated before surgery (7). At follow up of ovarian cancer after primary treatment, analysis of CA-125 and HE4 in combination with computed tomography (CT) scan is usually performed when suspicion of relapse.
In recent years, cell-free circulating tumor DNA (ctDNA) has emerged as a promising tumor biomarker (8). Through cell apoptosis, necrosis and other mechanisms of tumor cell death, small pieces of DNA are released into the bloodstream. These pieces are called cell-free circulating tumor DNA. ctDNA can be used for diagnosis, monitoring of treatment response and/or resistance, detection of latent disease, and prediction of outcome (8). It has been reported that elevated preoperative total plasma cell free DNA levels are correlated to events of advanced epithelial ovarian carcinoma (9). Advances in deep-sequencing technology enable comprehensive cataloguing of tumor-specific (somatic) chromosomal rearrangements and mutations, which can then be detected in follow-up plasma samples using mutation detection technologies (10).
The aim of the study was to measure tumor-specific mutations in plasma in ovarian cancer and borderline ovarian tumors after targeted sequencing of primary tumor tissues and to relate the ctDNA measurements to clinicopathological features and patient outcome. Tumor-specific mutations in ovarian tumors were identified by targeted sequencing, which informed ctDNA analyses in matched plasma samples using the ultrasensitive SAGAsafe digital PCR method.
Patients and Methods
Sample collection. Peripheral plasma samples were obtained preoperatively (the day before surgery or the same day) from 41 patients admitted for primary surgery of adnexal masses to the Department of Obstetrics and Gynecology in Lund, Sweden, between October 2004 and December 2012. Fresh frozen tumor biopsies from the same patients were also obtained and all samples stored at −80˚C until analyzed. The 41 patients were selected as a representative sample of ovarian tumor patients from the biobank. All data were grouped according to tumor type and stage of cancer: benign (B), borderline (BOT) and ovarian cancer (OC Stage I-IV).
Sequencing. DNA was extracted from tumor samples using the AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany) between 2018 and 2020. Tumor DNA samples were sheared to an average of 250 bp using a Covaris ultrasonicator before generating Illumina-compatible sequencing libraries using the KAPA HyperPrep Kit (Roche, Pleasanton, CA, USA). Total library yields were measured using Qubit (ThermoFisher, Cambridge, UK) and size distributions checked with BioAnalyzer (Agilent, Santa Clara, CA, USA) before adding equimolar amounts of each library into pools subjected to target enrichment. Targeted regions of the 41 libraries were hybridized according to the manufacturer’s protocol with the xGen Pan-Cancer Panel (Integrated DNA Technologies; IDT Inc, Coralville, IA, USA) containing 127 cancer-associated genes or a custom xGen Lockdown Probes panel covering exons and hotspots in 14 genes recurrently mutated in cancer (IDT). The hybridized libraries were sequenced using Illumina NextSeq 550 or MiSeq (Illumina Inc, San Diego, CA, USA).
Sequencing data was processed using a Snakemake workflow (11) and Bioconda (12) software packages. Basecalls were demultiplexed by sample and converted to unmapped BAM format using Picard v2.21.1, and unique molecular barcodes (UMIs) were extracted using fgbio v0.7.0. (https://github.com/fulcrumgenomics/fgbio) Reads with matching UMIs (allowing for a maximum 1 base mismatch) were collapsed to generate consensus reads. These were aligned to the hg38 version of the human reference genome using BWA v0.7.17 (13). Variants were called using VarDict-Java v1.7.0 (14). Annotation was performed using vcfanno v0.3.1 (15) and the data sources COSMIC v87 (16), dbSNP build 151 (17), as well as the Swedish and Danish human variation databases. To remove germline variants and sequencing artifacts, all variant calls that were also found in normal samples from five healthy donors were filtered, and only retained candidate variants as somatic that a) passed basic VarDict-Java criteria (“PASS” variants), b) were not in sequence repeat regions, and c) were not known common germline variants in the population according the dbSNP and COSMIC databases, and the Swedish and Danish variation data (defined as >1% population frequency).
Cell-free DNA was isolated from patient plasma samples using the QIAamp MinElute cfDNA kits (Qiagen). CA-125 was analyzed at the routine laboratory at Lund University Hospital with ELISA. For each patient, candidate somatic mutations were selected and SAGAsafe digital PCR (dPCR) assays or developed (SAGA Diagnostics AB, Lund, Sweden). All assays were validated using positive control tumor DNA and wild-type negative control DNA samples. One individual assay was selected for each patient. The SAGAsafe technology detects point mutations and small indels down to 0.001% variant allele frequency. These assays provide quantitative result in terms of mutant- and wild-type copy number and the mutant allele frequency (MAF) for an analyzed sample. The assays were developed and validated on the Bio-Rad QX200/QXDx Droplet Digital PCR System (Hercules, CA, USA).
Statistical analysis. One-way ANOVA with Bonferroni correction as post hoc test and trends across ordered groups were analyzed using linear regression with log-transformed values. Overall survival probabilities were calculated using the Kaplan–Meier method and the log-rank test. All comparisons were two-sided, and a 5% level of significance was used. The statistical analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA).
Ethics approval and consent to participate. Written informed consent was obtained from all study participants. Ethical approval was granted by the Ethical Review Board at the Faculty of Medicine, Lund University, Sweden. Dnr 495 2016 (amendment to Dnr 558-2004 and 94-2006).
Results
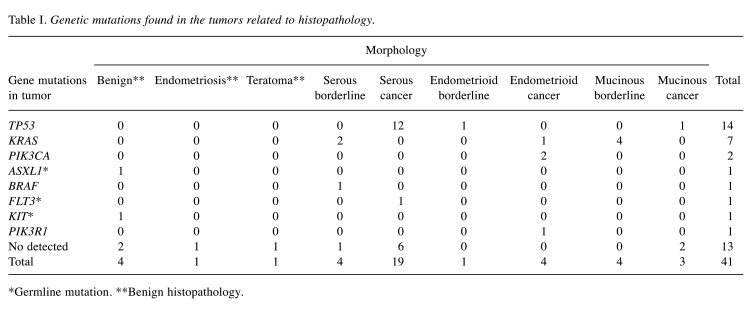
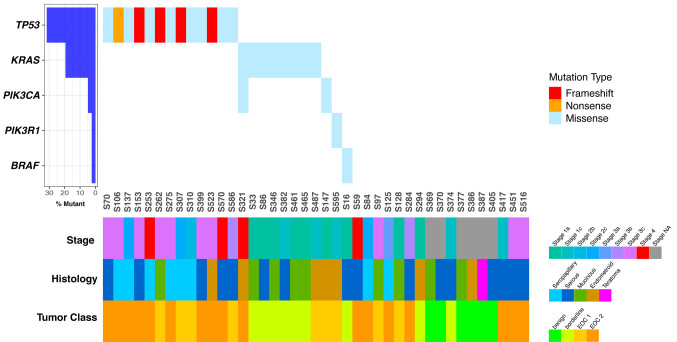
Patient data were grouped according to tumor type as follows: Benign (n=6), BOT (n=9) and ovarian cancers (n=26). The benign group generally consisted of younger patients (mean age=48 years ±13.3) compared to the cancer group (64 years ±11.9). Preoperative CA-125 levels in the BOT group were 293.7±627.3 units/ml and in the cancer group 1,264.3±1,147.1 units/ml. Somatic mutations were found in 24 tumor samples (seven were in BOT and 17 in cancer patients). TP53, KRAS, PIK3CA, PIK3R1, and BRAF were the most recurrently mutated genes in this cohort (Table I and Figure 1). In stage III ovarian cancer, there were eight tumors with a TP53 mutation and one with PIK3R1 mutations (Figure 1). KRAS mutations were found in four mucinous borderline tumors and two serous borderline tumors.
Table I. Genetic mutations found in the tumors related to histopathology.
*Germline mutation. **Benign histopathology.
Figure 1. Waterfall plot of validated somatic mutations in patient tumors. Genes are indicated in rows, and samples in columns. Mutated samples are shown according to mutation type. Patient and tumor clinicopathological variables are shown below the patient IDs.
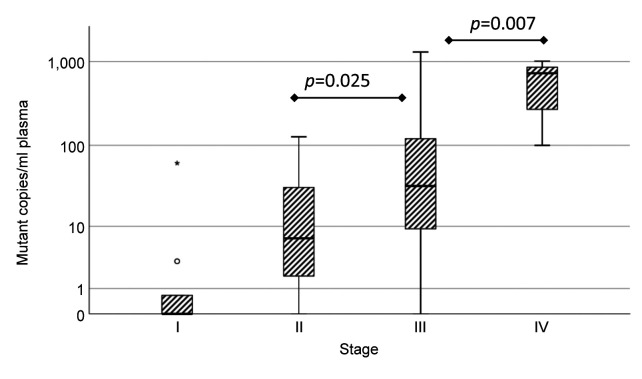
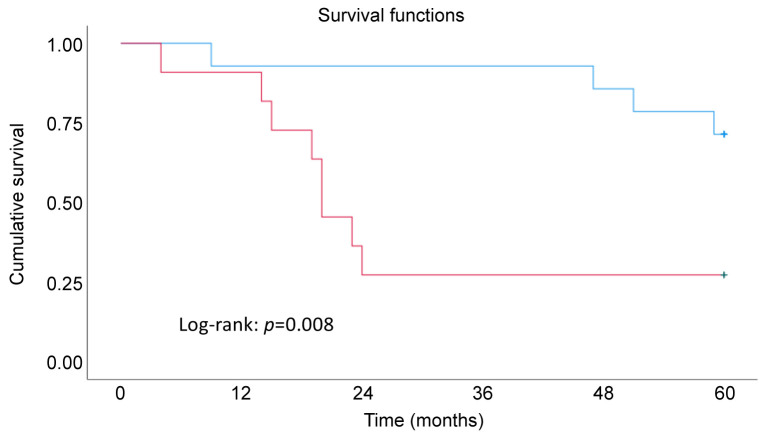
The mutations detected in plasma were in TP53, KRAS, PIK3CA, and PIK3R1. Seven patients with stage III, three with stage IV and four with stages I and II of ovarian cancer had detectable ctDNA in the plasma. All serous cancer patients with detectable ctDNA showed TP53 mutations, and one serous borderline patient had a KRAS mutation in plasma. The concentration of ctDNA increased with higher stage (p for trend <0.001). Concentrations of ctDNA in stage III and IV were significantly higher compared with stage I (p=0.025 and 0.007, respectively) (Figure 2). The number of 10 mutant copies per ml for the survival analyses was chosen after studying the results (Figure 2), in which the number of mutant copies is shown related to stage. Cancer patients with more than 10 mutant copies/ml in plasma showed significantly worse overall survival (OS, p=0.008 by log-rank test) (Figure 3).
Figure 2. Plasma circulating tumor DNA (ctDNA) mutant concentration increased with higher stage (p for trend <0.001). Bars show the highest and lowest values, except outliers (◯), which are 1.5 to 3 box lengths from the end of the box, and extremes (*) which are more than 3 box lengths from the end of the box. Concentrations of ctDNA in stage III and IV were significantly higher compared with stage I (p=0.025 and 0.007, respectively). One-way ANOVA with Bonferroni correction and trends across ordered groups were analyzed using linear regression with log-transformed values.
Figure 3. Kaplan Meier analysis of overall survival in patients related to genetic mutation found in plasma (OS, p=0.008 by log-rank test). The blue line corresponds to <10 copies/ml and the red line >10 copies/ml. Among patients with ctDNA levels below 10 copies/ml, 2 out of 3 experienced mortality, while among those with ctDNA levels exceeding 10 copies/ml, 10 out of 11 patients faced mortality.
Discussion
In this study, ctDNA was measured in plasma samples from ovarian cancer patients and borderline ovarian tumor patients using the SAGAsafe dPCR technology. The plasma levels of ctDNA mutations were increased in patients with higher stage of disease. In patients with ovarian cancer, higher levels of ctDNA in plasma were associated with worse OS. These results indicate that ctDNA analyzed in plasma can be useful pre-operatively both as a diagnostic and as a prognostic biomarker in ovarian cancer.
Specific genomic alterations in ctDNA can guide cancer treatment. The most frequently mutated gene in these patients with ovarian tumors was TP53, especially in advanced stage OC. Several studies have shown that TP53 mutations are significantly associated with advanced OC since they are found in more than 90% of the high-grade serous ovarian carcinomas (18). Loss of p53 function makes cells unable to induce apoptosis and therefore primes these cells for transformation into malignancy (19,20). Most mutations are single-base substitutions distributed throughout the coding sequence. TP53 mutations have been shown to be prognostic and targets for pharmacological intervention (21). In the current Swedish national guidelines (RCC), PARP inhibitors are recommended for use in treatment of BRCA-mutated epithelial ovarian cancer (22). PARP inhibitors have demonstrated efficacy in BRCA-mutated ovarian cancer (22) as well as in other malignancies characterized by homologous recombination deficiency. Only a few studies have been performed to evaluate the efficacy of PARP inhibitors in terms of TP53 status (23), and it has been presumed that targeting mutant p53, which destabilizes PARP repair function, may impede the distant spread of cancer cells (24).
KRAS gene mutations were found in seven tissue specimens, and one was found in plasma ctDNA. KRAS is a member of the Ras family of oncogenes, which also includes two other genes: HRAS and NRAS. The proteins these genes encode play important roles in cell division, cell differentiation, and apoptosis. Six mutations in KRAS were found in borderline tumors. Low-grade serous carcinomas (LGSOC) may progress from borderline tumors with frequent mutations of the KRAS, BRAF, or ERBB2 genes and lack of TP53 mutations (25). Two multicenter trials studying MEK inhibitors in LGSOC demonstrated activity in LGSOC, especially in KRAS-mutated disease. Accordingly, MEK inhibitors could be an alternative treatment in LGSOC (26). In patients with advanced malignant melanoma with BRAF mutations, KRAS inhibition (KRASi) has shown good results (27). In lung adenocarcinoma patients with co-occurring TP53 mutations, clinical benefit has been demonstrated from PD-1 inhibitors and mutation status may guide anti-PD-1/PD-L1 immunotherapy (28).
Concordance between tumor DNA and ctDNA. Various plasma ctDNA technologies are being tested in academic and commercial laboratories (29). Plasma ctDNA testing has a promising role in follow up as a tumor marker, especially when treatment is based or monitored on a gene mutation profile (30). In this study, it was found that 15 out of 24 patients with tumor DNA mutations had mutations detected in plasma ctDNA, which corresponds to a sensitivity of 62.5%, in line with previous reports (31). However, the possibility to detect ctDNA mutations in plasma depends on the morphology of the tumor, the stage of the disease, tumor burden, as well as DNA degradation (31).
ctDNA in relation to stage. The plasma ctDNA mutant concentration increased with higher stage, which is in accordance with previous studies (32). In late-stage cancer, especially in abdominal organs, such as colon, pancreas, or ovaries, ctDNA has been commonly detected in more than 60% of patients (33). In this study ctDNA was detected in two patients in stage I OC. Interestingly, our study also detected ctDNA mutation in plasma in one patient with a borderline tumor, which has not previously been reported. Other studies observed that tumor volume assessed by CT imaging correlated with ctDNA levels in patients with relapsed high-grade serous ovarian cancer (34). Patients with advanced ovarian cancer have had median concentrations of 100–1,000 mutated gene copies per 5 ml of plasma (34). The analyses in this study were able to detect less than 10 copies/ml in plasma in the early stage of the disease, indicating very high sensitivity. The quantity of ctDNA in plasma correlates with tumor size and disease stage, as indicated by our observations and previous research (35).
ctDNA mutations/ml plasma as a prognostic factor. Confirming the aim of our study, that it is possible to detect ctDNA in ovarian cancer patients, we can further conclude that our results showed that patients in advanced stage of OC had more mutated gene copies than in early stage of disease. The prognosis for ovarian cancer patients is highly related to stage (36). Similar studies with gynecologic cancers have found that higher percentage of ctDNA correlated with worse survival (37). In colorectal cancers ctDNA has found to be a prognostic factor for disease recurrence (38), and another study identified ctDNA as a potential independent factor (39). In addition, higher ctDNA concentration significantly correlated with worse progression-free survival (PFS) (40). Our results showed that patients with more than 10 ctDNA mutation copies/ml plasma had significantly worse overall survival.
Strengths and limitations. The findings of this study have demonstrated the potential clinical utility of ctDNA analysis in detecting ovarian cancer and predicting survival. However, it is important to note that the study was conducted on a relatively small patient cohort and only genetic variants in the coding regions of genes were investigated. Previous studies have found that variants located in the intronic, and non-coding regions of genomes may play a role in identifying individuals at genetic risk of developing ovarian cancer (41). This and other sequencing studies have limitations in identifying genetic variants associated with OC in non-coding regions. The presence of ctDNA in the bloodstream is directly proportional to the tumor burden in the body, and patients with a larger tumor burden are more likely to have detectable ctDNA. The sensitivity of ctDNA detection can be influenced by the technology employed for genetic analysis. In this study, high-sensitivity sequencing data and coverage were used to detect ctDNA. The quality and quantity of ctDNA can also be influenced by other factors, such as age and overall health status. For example, ctDNA levels may be higher in older patients due to the natural aging process, while patients with other medical conditions or treatments may have lower levels.
Implications for practice and future research. The study demonstrated the potential clinical utility of ctDNA analysis in detecting ovarian cancer and predicting survival, particularly in advanced stages. However, the results need to be validated in larger patient cohorts before clinical implementation. Further research is needed to explore the detection of genetic variants in non-coding regions and improve the accuracy of ovarian cancer detection using ctDNA analysis. The concentration of ctDNA mutated copies in plasma was found to be related to stage and overall survival, suggesting its potential as a prognostic tool; in larger studies, the optimal cut-off level of mutant copies per ml should be evaluated. In this study, candidate somatic mutations were sequenced from the available tumor material for ctDNA analysis in plasma. In cases where tumor material is not available, broad genomic panels must be designed for ctDNA analysis in plasma. The future use of broader genomic panels may simplify the ctDNA analyses excluding the need for high-sensitivity DNA sequencing analyses of tumor tissue, retaining the high sensitivity of ctDNA analysis and potentially improving the accuracy of OC detection.
Conclusion
This study found that ctDNA mutations could be detected in 15 out of 24 patients with ovarian cancer and pre-malignant ovarian borderline tumors. The most mutated gene was TP53. The concentration of ctDNA mutated copies in plasma was related to stage and was higher in advanced OC stages. Patients with an increased amount of ctDNA in plasma experienced worse overall survival. Plasma is easily accessible and ctDNA may be used for prediction of prognosis.
Conflicts of Interest
The Authors are solely responsible for the content and writing of the paper. Lao H Saal has received honoraria from Novartis and Boehringer-Ingelheim. Yilun Chen, Christian Brueffer and Lao H Saal have ownership interests in SAGA Diagnostics. The Authors declare that they have no other conflicts of interest.
Authors’ Contributions
AD: Study conception and design; data acquisition, analysis, and interpretation; drafting and revising the manuscript. PL: Data acquisition and revising the manuscript. YC: Data acquisition, analysis, and interpretation; drafting and revising the manuscript. ChB: Data acquisition, analysis, and interpretation; drafting and revising the manuscript. LHS: Study conception and design; data acquisition, analysis, and interpretation; revising the manuscript. ChBo: Study conception and design; data acquisition, analysis, and interpretation; revising the manuscript.
Acknowledgements
We are grateful to all the patients who participated in this study, to the staff at the Department of Obstetrics and Gynecology, Lund University Hospital, for assistance with collecting the blood samples and to the laboratory staff at the Division of Oncology. The study was supported by funds from the Swedish Cancer Foundation and Regional Funds Region Skåne. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.International agency for research on cancer, 2020. Available at: https://gco.iarc.fr/today/fact-sheets-cancers. [Last accessed on March 10, 2022]
- 2.Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JWW. Recent trends of cancer in Europe: A combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44(10):1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi: 10.1016/s0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 4.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B, Crawford ED, Church TR, Andriole GL, Weissfeld JL, Fouad MN, Chia D, O’Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hartge P, Pinsky PF, Zhu CS, Izmirlian G, Kramer BS, Miller AB, Xu JL, Prorok PC, Gohagan JK, Berg CD, PLCO Project Team. Effect of screening on ovarian cancer mortality. JAMA. 2011;305(22):2295. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E, Cruickshank D, Crump DN, Davies SK, Dawnay A, Dobbs S, Fletcher G, Ford J, Godfrey K, Gunu R, Habib M, Hallett R, Herod J, Jenkins H, Karpinskyj C, Leeson S, Lewis SJ, Liston WR, Lopes A, Mould T, Murdoch J, Oram D, Rabideau DJ, Reynolds K, Scott I, Seif MW, Sharma A, Singh N, Taylor J, Warburton F, Widschwendter M, Williamson K, Woolas R, Fallowfield L, McGuire AJ, Campbell S, Parmar M, Skates SJ. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, Carlino G, Taylor J, Massingham SK, Raikou M, Kalsi JK, Woolas R, Manchanda R, Arora R, Casey L, Dawnay A, Dobbs S, Leeson S, Mould T, Seif MW, Sharma A, Williamson K, Liu Y, Fallowfield L, McGuire AJ, Campbell S, Skates SJ, Jacobs IJ, Parmar M. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182–2193. doi: 10.1016/S0140-6736(21)00731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsen MA, Høgdall EV, Christensen IJ, Borgfeldt C, Kalapotharakos G, Zdrazilova-dubska L, Chovanec J, Lok CA, Stiekema A, Mutz-dehbalaie I, Rosenthal AN, Moore EK, Schodin BA, Sumpaico WW, Sundfeldt K, Kristjansdottir B, Zapardiel I, Høgdall CK. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer — An international multicenter study in women with an ovarian mass. Gynecol Oncol. 2015;138(3):640–646. doi: 10.1016/j.ygyno.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MH, Dahlgren M, Schulz R, Grabau D, van Westen D, Fernö M, Ingvar C, Rose C, Bendahl PO, Rydén L, Borg Å, Gruvberger-Saal SK, Jernström H, Saal LH. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015;7(8):1034–1047. doi: 10.15252/emmm.201404913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamat AA, Baldwin M, Urbauer D, Dang D, Han LY, Godwin A, Karlan BY, Simpson JL, Gershenson DM, Coleman RL, Bischoff FZ, Sood AK. Plasma cell-free DNA in ovarian cancer: an independent prognostic biomarker. Cancer. 2010;116(8):1918–1925. doi: 10.1002/cncr.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Förnvik D, Aaltonen KE, Chen Y, George AM, Brueffer C, Rigo R, Loman N, Saal LH, Rydén L. Detection of circulating tumor cells and circulating tumor DNA before and after mammographic breast compression in a cohort of breast cancer patients scheduled for neoadjuvant treatment. Breast Cancer Res Treat. 2019;177(2):447–455. doi: 10.1007/s10549-019-05326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köster J, Rahmann S. Snakemake – a scalable bioinformatics workflow engine. Bioinformatics. 2012;28(19):2520–2522. doi: 10.1093/bioinformatics/bts480. [DOI] [PubMed] [Google Scholar]
- 12.Grüning B, Dale R, Sjödin A, Chapman BA, Rowe J, Tomkins-Tinch CH, Valieris R, Köster J, Bioconda Team Bioconda: sustainable and comprehensive software distribution for the life sciences. Nat Methods. 2018;15(7):475–476. doi: 10.1038/s41592-018-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai Z, Markovets A, Ahdesmaki M, Chapman B, Hofmann O, McEwen R, Johnson J, Dougherty B, Barrett JC, Dry JR. VarDict: a novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res. 2016;44(11):e108. doi: 10.1093/nar/gkw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen BS, Layer RM, Quinlan AR. Vcfanno: fast, flexible annotation of genetic variants. Genome Biol. 2016;17(1):118. doi: 10.1186/s13059-016-0973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sondka Z, Bamford S, Cole CG, Ward SA, Dunham I, Forbes SA. The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat Rev Cancer. 2018;18(11):696–705. doi: 10.1038/s41568-018-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Cao L, Nguyen D, Lu H. TP53 mutations in epithelial ovarian cancer. Transl Cancer Res. 2016;5(6):650–663. doi: 10.21037/tcr.2016.08.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozaki T, Nakagawara A. Role of p53 in cell death and human cancers. Cancers (Basel) 2011;3(1):994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser AM, Attardi LD. Deconstructing networks of p53-mediated tumor suppression in vivo. Cell Death Differ. 2018;25(1):93–103. doi: 10.1038/cdd.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2(1):a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore K, Colombo N, Scambia G, Kim B, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza A, González-Martín A, Aghajanian C, Bradley W, Mathews C, Liu J, Lowe ES, Bloomfield R, DiSilvestro P. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 23.Collot T, Niogret J, Carnet M, Chevrier S, Humblin E, Favier L, Bengrine-Lefevre L, Desmoulins I, Arnould L, Boidot R. PARP inhibitor resistance and TP53 mutations in patients treated with olaparib for BRCA-mutated cancer: Four case reports. Mol Med Rep. 2020;23(1):75. doi: 10.3892/mmr.2020.11713. [DOI] [PubMed] [Google Scholar]
- 24.Corcoran NM, Clarkson MJ, Stuchbery R, Hovens CM. Molecular pathways: Targeting DNA repair pathway defects enriched in metastasis. Clin Cancer Res. 2016;22(13):3132–3137. doi: 10.1158/1078-0432.Ccr-15-1050. [DOI] [PubMed] [Google Scholar]
- 25.Vang R, Shih IeM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16(5):267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauly N, Ehmann S, Ricciardi E, Ataseven B, Bommert M, Heitz F, Prader S, Schneider S, Du Bois A, Harter P, Baert T. Low-grade serous tumors: are we making progress. Curr Oncol Rep. 2020;22(1):8. doi: 10.1007/s11912-020-0872-5. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich P, Kuphal S, Spruss T, Hellerbrand C, Bosserhoff AK. Wild-type KRAS is a novel therapeutic target for melanoma contributing to primary and acquired resistance to BRAF inhibition. Oncogene. 2018;37(7):897–911. doi: 10.1038/onc.2017.391. [DOI] [PubMed] [Google Scholar]
- 28.Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, Yang JJ, Yang XN, Lin JX, Yan HH, Zhai HR, Yan LX, Liao RQ, Wu SP, Wu YL. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–3024. doi: 10.1158/1078-0432.CCR-16-2554. [DOI] [PubMed] [Google Scholar]
- 29.Cheng ML, Pectasides E, Hanna GJ, Parsons HA, Choudhury AD, Oxnard GR. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA Cancer J Clin. 2021;71(2):176–190. doi: 10.3322/caac.21650. [DOI] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 31.Bieg-Bourne CC, Okamura R, Kurzrock R. Concordance between TP53 alterations in blood and tissue: impact of time interval, biopsy site, cancer type and circulating tumor DNA burden. Mol Oncol. 2020;14(6):1242–1251. doi: 10.1002/1878-0261.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liebs S, Keilholz U, Kehler I, Schweiger C, Haybäck J, Nonnenmacher A. Detection of mutations in circulating cell-free DNA in relation to disease stage in colorectal cancer. Cancer Med. 2019;8(8):3761–3769. doi: 10.1002/cam4.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, Antonarakis ES, Azad NS, Bardelli A, Brem H, Cameron JL, Lee CC, Fecher LA, Gallia GL, Gibbs P, Le D, Giuntoli RL, Goggins M, Hogarty MD, Holdhoff M, Hong SM, Jiao Y, Juhl HH, Kim JJ, Siravegna G, Laheru DA, Lauricella C, Lim M, Lipson EJ, Marie SK, Netto GJ, Oliner KS, Olivi A, Olsson L, Riggins GJ, Sartore-Bianchi A, Schmidt K, Shih lM, Oba-Shinjo SM, Siena S, Theodorescu D, Tie J, Harkins TT, Veronese S, Wang TL, Weingart JD, Wolfgang CL, Wood LD, Xing D, Hruban RH, Wu J, Allen PJ, Schmidt CM, Choti MA, Velculescu VE, Kinzler KW, Vogelstein B, Papadopoulos N, Diaz LA Jr. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 35.Ogasawara A, Hihara T, Shintani D, Yabuno A, Ikeda Y, Tai K, Fujiwara K, Watanabe K, Hasegawa K. Evaluation of circulating tumor DNA in patients with ovarian cancer harboring somatic PIK3CA or KRAS mutations. Cancer Res Treat. 2020;52(4):1219–1228. doi: 10.4143/crt.2019.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cancer stat facts: Ovarian cancer, 2020. Available at: https://seer.cancer.gov/statfacts/html/ovary.html. [Last accessed on March 24, 2022]
- 37.Charo LM, Eskander RN, Okamura R, Patel SP, Nikanjam M, Lanman RB, Piccioni DE, Kato S, McHale MT, Kurzrock R. Clinical implications of plasma circulating tumor DNA in gynecologic cancer patients. Mol Oncol. 2021;15(1):67–79. doi: 10.1002/1878-0261.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benhaim L, Bouché O, Normand C, Didelot A, Mulot C, Le Corre D, Garrigou S, Djadi-Prat J, Wang-Renault S-F, Perez-Toralla K, Pekin D, Poulet G, Landi B, Taieb J, Selvy M, Emile J-F, Lecomte T, Blons H, Chatellier G, Link DR, Taly V, Laurent-Puig P. Circulating tumor DNA is a prognostic marker of tumor recurrence in stage II and III colorectal cancer: multicentric, prospective cohort study (ALGECOLS) Eur J Cancer. 2021;159:24–33. doi: 10.1016/j.ejca.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Taieb J, Taly V, Vernerey D, Bourreau C, Bennouna J, Faroux R, Desrame J, Bouche O, Borg C, Egreteau J, Mineur L, Lepere C, Deplanque G, Mulot C, Louvet C, Mabro M, Ychou M, de Gramont A, Andre T, Laurent-Puig P. Analysis of circulating tumour DNA (ctDNA) from patients enrolled in the IDEA-FRANCE phase III trial: Prognostic and predictive value for adjuvant treatment duration. Ann Oncol. 2019;30:v867. doi: 10.1093/annonc/mdz394.019. [DOI] [Google Scholar]
- 40.Noguchi T, Iwahashi N, Sakai K, Matsuda K, Matsukawa H, Toujima S, Nishio K, Ino K. Comprehensive gene mutation profiling of circulating tumor DNA in ovarian cancer: its pathological and prognostic impact. Cancers (Basel) 2020;12(11):3382. doi: 10.3390/cancers12113382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Paranjape T, Stahlhut C, McVeigh T, Keane F, Nallur S, Miller N, Kerin M, Deng Y, Yao X, Zhao H, Weidhaas JB, Slack FJ. Targeted resequencing of the microRNAome and 3’UTRome reveals functional germline DNA variants with altered prevalence in epithelial ovarian cancer. Oncogene. 2015;34(16):2125–2137. doi: 10.1038/onc.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]