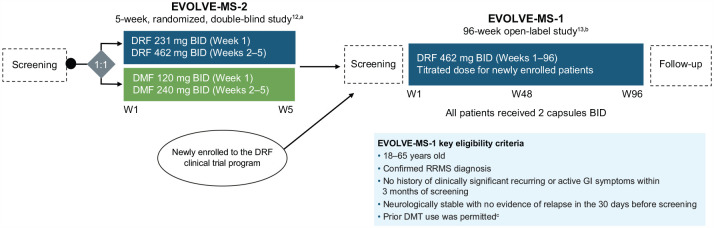
Figure 1.
EVOLVE-MS-1 study design.
BID: twice daily; DMF: dimethyl fumarate; DMT: disease-modifying therapy; DRF: diroximel fumarate; GI: gastrointestinal; RRMS: relapsing–remitting multiple sclerosis; W: week.
EVOLVE-MS-1 was conducted from 10 December 2015 to 11 November 2021.
aAdapted from Naismith RT, et al. CNS Drugs. 2020;34(2):185–196; http://creativecommons.org/licenses/by-nc/4.0/.
bAdapted from Naismith RT, et al. Mult Scler. 2020;26(13):1729–1739; http://creativecommons.org/licenses/by-nc/4.0/.
cExclusion criteria for newly enrolled patients included the use of teriflunomide within 2 years of Visit 2 (Week 1); natalizumab within 2 months of Visit 2; alemtuzumab; fingolimod within 90 days of Visit 2; daclizumab within 6 months of Visit 2; or B-cell therapies within 12 months of screening.