Abstract
Background:
Blood cultures are overused in pediatric ICUs (PICUs), which may lead to unnecessary antibiotic use and antibiotic resistance. Using a participatory ergonomics (PE) approach, we disseminated a quality improvement (QI) program for optimizing blood culture use in PICUs to a national 14-hospital collaborative. The objective of this study was to evaluate the dissemination process and its impact on blood culture reduction.
Methods:
The PE approach emphasized three key principles (i.e., stakeholder participation, application of human factors and ergonomics knowledge and tools, cross-site collaboration) with a six-step dissemination process. Data on interactions between sites and the coordinating team and site experiences with the dissemination process were collected using site diaries and semi-annual surveys with local QI teams, respectively, and correlated with the site-specific change in blood culture rates.
Results:
Overall, participating sites were able to successfully implement the program and reduced their blood culture rates from 149.4 blood cultures per 1000 patient-days/month before implementation to 100.5 blood cultures per 1000 patient-days/month after implementation, corresponding to a 32.7% relative reduction (p < 0.001). Variations in the dissemination process, as well as in local interventions and implementation strategies, were observed across sites. Site-specific changes in blood culture rates were weakly negatively correlated with the number of pre-intervention interactions with the coordinating team (p = 0.057), but not correlated with their experiences with the six domains of the dissemination process or their interventions.
Conclusions:
We applied a PE approach to disseminate a QI program for optimizing PICU blood culture use to a multi-site collaborative. Working with local stakeholders, participating sites tailored their interventions and implementation processes and achieved the goal of reducing blood culture use.
Keywords: quality improvement, dissemination, participatory ergonomics, blood culture
INTRODUCTION
Over the past two decades, substantial efforts and resources have been devoted to improving the quality and safety of health care.1–3 The dissemination of successful quality improvement (QI) programs to a broader range of health care settings, however, faces different challenges (for example, engagement of local stakeholders, adaptation of QI programs to local context).4 While various frameworks with dissemination principles and processes have been proposed,5–7 empirical research on effective QI dissemination (for example, multi-center QI initiatives) is limited.8
We initiated a QI program for optimizing blood culture use in pediatric ICUs (PICUs) at Johns Hopkins Hospital (JHH)9 and pilot-tested it at two additional hospitals.10 The QI program was then disseminated to a national 14-hospital collaborative using a participatory ergonomics (PE) approach.11 Results on the effectiveness of the QI program across the collaborative were reported by Woods-Hill et al. in 2022.12 In the present paper, we evaluate the dissemination process and its impact on blood culture reduction. The Johns Hopkins University School of Medicine Institutional Review Board approved this study.
The BrighT STAR (Testing STewardship for Antibiotic Reduction) Collaborative
Blood cultures are a key diagnostic test for assessing bacterial bloodstream infection, a common condition in PICUs that can lead to significant morbidity and mortality.13 Perceived as a low-risk test for a disease with disastrous outcomes, blood cultures are excessively used in PICUs,14 and false positive blood culture results may lead to additional laboratory tests, unnecessary antibiotic use, prolonged hospitalization, and increased health care costs.15, 16 In 2014, we developed and implemented a QI program in the PICU at JHH to optimize blood culture use. As part of the program, a paper-based clinical decision support (CDS) tool was developed to guide clinicians to consider possible alternative sources of infection (such as surgical site infection), evaluate non-infectious sources of fever (for example, opioid withdrawal), and carefully review risk factors of patients for bloodstream infections (for example, compromised immune system). The implementation of the program resulted in a reduction in the total number of blood cultures collected by 45.6% and in the percentage of blood cultures collected from central venous catheters from 73.1% to 39.5%, without an increased number of episodes of suspected infection and septic shock or a decreased proportion of suspected septic shock occurrences with a blood culture obtained.9 The program was then adopted by two additional hospitals, who achieved a 27.8% to 51.9% reduction in blood culture use.10 Given the initial success of the program, a multi-site collaborative called BrighT STAR was created to scale-up the program and assess its broader impact on blood culture use, antibiotic use, and patient outcomes.12
Overall Approach for Dissemination.
PE, a macroergonomic approach to promote the application of human factors and ergonomics to work system design, emphasizes the involvement of people in “planning and controlling a significant amount of their own work activities, with sufficient knowledge and power to influence both processes and outcomes in order to achieve desirable goals.”11 A PE approach can vary along different dimensions, including permanency of the initiative, nature of involvement, level of influence, location of decision-making power, mix of participants, requirement of involvement, topics to be addressed, extent of involvement, and role of ergonomics specialists.17 While PE has been applied across a range of industries (for example, manufacturing,18, 19 construction,20, 21 mining22), its application in health care has been limited, focusing on task and process design in individual health care settings.23, 24
In this study, we broaden the application of PE to facilitate the dissemination of the program to the BrighT STAR collaborative. Two key PE principles were emphasized: (1) participation of different stakeholders in work system and process redesign and (2) application of human factors and ergonomics (HFE) knowledge and tools to work system and process redesign. The adaptation and implementation of the program at individual sites involved local stakeholders who could affect or be affected by the blood culture ordering process and applied different HFE design principles (for example, systems approach, shared mental model, usability) and implementation principles (for example, leadership support, stakeholder engagement, communication and feedback, learning and training, project management).25 In addition, given the nature of the BrighT STAR collaborative, cross-site collaboration was emphasized to facilitate peer-to-peer learning26 and create isomorphic pressures.27
Structure of the BrighT STAR collaborative.
The BrighT STAR collaborative was guided by the coordinating team developing the program at JHH, which consisted of one pediatric infectious disease physician, two pediatric intensivists, one human factors engineer, one biostatistician, and three program coordinators. Fourteen teaching hospitals were recruited to participate in the BrighT STAR collaborative (Table 1). Each participating hospital convened a local QI team (Dissemination Process – Step 1), which worked with local stakeholders to develop and implement their own interventions, including CDS tools and other changes to the work systems (for example, institutional policy) and processes (such as daily rounds or huddles). The local QI teams routinely communicated with the coordinating team through various avenues, including an initial webinar, monthly individual site calls, monthly all-site calls, individual and group emails, and individual phone calls.
Table 1.
Baseline Site Characteristics
| Characteristics | Sites |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Region | W | W | NE | SE | SE | NE | MW | W | SE | MW | SW | MW | W | MW |
| Number of PICU beds | 20 | 28 | 60 | 12 | 38 | 30 | 21 | 36 | 52 | 25 | 24 | 40 | 42 | 40 |
| Annual admissions | 1,240 | 2,000 | 4,011 | 350 | 1,600 | 2,035 | 1,680 | 1,940 | 4,057 | 1,500 | 1,554 | 1,700 | 2,400 | 2,000 |
| Annual patient days | 4,746 | 10,000 | 20,650 | 2,000 | 5,352 | 9,711 | 5,600 | 7,801 | 14,859 | 6,250 | 4,552 | 13,000 | 10,843 | 10,258 |
| Baseline blood culture rates* | 166.2 | 182.8 | 102.1 | 386.8 | 98.0 | 151.1 | 142.9 | 105.1 | 74.8 | 125.2 | 171.7 | 186.4 | 480.5 | 200.0 |
Mean monthly number of blood cultures per 1000 patient-days.
W, West; NE, Northeast; SE, Southeast; MW, Midwest; SW, Southwest; PICU, pediatric ICU.
Dissemination Process.
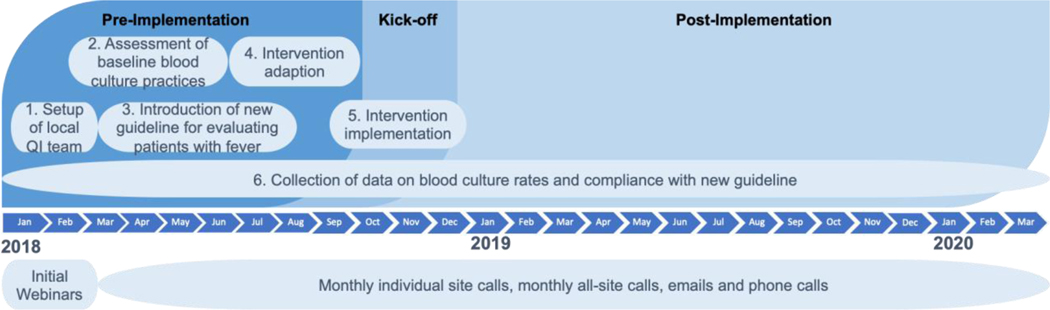
The dissemination process consisted of six steps over a period of 27 months (Figure 1). Table 2 describes each step of the dissemination process in detail.
Figure 1.
Shown here is the dissemination timeline for the collaborative.
Table 2.
Dissemination Process
| Steps | Descriptions |
|---|---|
| Step 1: Setup of local quality improvement (QI) teams | An initial webinar was held in early 2018 with the physician champions from all hospitals to review the background, development, and approaches of the program for optimizing blood culture use and discuss the goals, timeline, and expectations of the Bright STAR collaborative. After the initial webinar, hospitals confirmed their decision to participate in the collaborative, obtained approval from their local institutional review boards, and convened their local QI teams. The local QI team at each participating hospital was led by two physician champions (a pediatric critical care physician and a pediatric infectious disease physician) and involved representatives of other stakeholders (for example,, fellow physicians, nursing leaders, infection preventionists). Five hospitals (sites 6, 11, 12, 13, 14 in Table 1) also included a trained QI specialist on their team. |
| Step 2: Assessment of baseline blood culture practices | After convening local QI teams, participating hospitals completed a survey-based work system assessment, which aimed to (1) understand the current blood culture ordering practices, (2) examine beliefs about blood culture ordering practices, and (3) identify potential barriers to reducing unnecessary blood cultures.1 Based on the Systems Engineering Initiative for Patient Safety (SEIPS) 2.0 model2 and pilot studies at three hospitals, a 50-item survey was devised and administered across the collaborative using Qualtrics survey software (Qualtrics Labs Inc., Provo, Utah). The coordinating team summarized and shared the survey results with each local QI team during a dedicated individual site call. The ensuing discussion and sharing of insights into the results promoted a dialogue of how to use the survey results to facilitate program implementation. Following the call, the coordinating team sent a copy of the site-specific slide presentation along with a summary of the discussion to the local QI team. Finally, the aggregated survey results reflecting responses across the collaborative were shared with participating hospitals during an all-site call. |
| Steps 3 and 4: Introduction of a new clinical approach for evaluating patients with fever and local intervention development | Following the survey-based work system assessment, the coordinating team introduced the new clinical approach for evaluating patients with fever developed at JHH to local QI teams during a monthly all-site call. Local QI teams then worked with their local stakeholders to prioritize areas for improvement (for example, eliminating surveillance culturing, reviewing clinical data, and performing a physical exam before ordering a blood culture), customize intervention ideas (for example, fever checklist and blood culture collection algorithm, patient and family education materials), and formalize implementation strategies (for example, clinician training, audit and feedback). The coordinating team held monthly all-site calls to provide general guidance on intervention development (for example, conceptual frameworks, human factors and ergonomics design and implementation principles, examples of interventions and implementation plans) and encourage participating hospitals to share their experiences with each other. |
| Step 5: Local intervention implementation | Based on the preparation of the intervention and the execution of the implementation strategies, the QI team at each participating hospital determined their readiness for implementation and selected a kick-off date to officially launch their intervention. While the kick-off dates of most participating hospitals were between August 2018 and February 2019, one hospital experienced significant delay and launched their intervention in August 2019. The coordinating team continued to hold monthly individual site calls and sent bi-weekly check-in emails to help participating hospitals address their local implementation challenges. |
| Step 6: Collection of data on blood culture rates and compliance with new clinical approach | Data on blood culture rates (number of blood cultures collected per 1000 patient days) and other safety metrics (such as delay in blood culture collection) were collected monthly from each participating hospital throughout the dissemination process. The kick-off date served as a formal marker to separate pre- and post-intervention data. In addition, participating hospitals were provided with an audit tool to keep track of adherence to their CDS tool at regular intervals during the post-intervention phase. The coordinating team continuously communicated with participating hospitals through monthly individual site calls, monthly all-site calls, and individual and group emails. |
METHODS
A mixed methods study28 was conducted to evaluate the dissemination process and its impact on site performance. In addition to quantitative data on blood culture use (Dissemination Process Step 6, Table 2), interactions between participating hospitals and the coordinating team were qualitatively recorded using site diaries and quantitatively summarized using frequencies; site experiences with the dissemination process were quantitatively assessed using semi-annual surveys with local QI teams. Data collected by site diaries and semi-annual surveys were correlated with the site-specific change in blood culture use.
Data Collection
Site Diaries.
To characterize the dissemination process, the coordinating team kept a diary for each participating hospital. Two program coordinators recorded real-time interactions (for example, emails, phone calls, conference calls) between each site and the coordinating team. Each entry included the date, purpose of the interaction, participants, and outcomes. Entries were jointly reviewed by the two coordinators on a weekly basis to ensure consistency.
Semi-annual Surveys.
Four surveys were conducted during individual site calls with local QI teams over time to examine their experiences with the dissemination process. A pre-implementation survey was conducted around each site’s kick-off with subsequent surveys approximately every six months. The four surveys consisted of similar questions assessing six domains of the dissemination process, including (1) leadership support (for example, How supportive is your unit leadership of the blood culture program?), (2) resources available (for example, How sufficient are the resources allocated for quality improvement in your ICU?), (3) engagement of champions (for example, To what extent is your blood culture program driven by individual champions who are influential on the unit?), (4) engagement of frontline staff (for example, How engaged is your frontline staff in the blood culture program?), (5) impact of the larger collaborative (for example, How useful has being part of the larger collaborative network been in facilitating your blood culture program?), and (6) impact of ongoing QI projects (for example, To what extent is your blood culture program hindered by ongoing quality improvement projects with competing goals?). All questions were assessed using 5-point Likert scales, with 5 representing the most positive response and 1 the most negative response. For each question, members of the local QI team were asked to discuss their opinions, achieve consensus, and provide a specific answer along the Likert scale. A copy of the pre-implementation survey is included in Appendix A. Minor revisions of the questions were made to align the surveys with the time point at which they were conducted.
Data Analysis
Site Diaries.
A qualitative content analysis29 of the site diaries was conducted. While conference calls included all-site calls and individual site calls, interactions through emails and phone calls were classified based on their purposes into five sub-categories: (1) data dialogue (for example, collecting monthly data on blood culture use and other clinical outcomes), (2) collection of site QI activities (for example, reminding or receiving site log detailing QI activities), (3) implementation facilitation (for example, addressing site questions about intervention design and implementation), (4) project administration (for example, providing support on regulatory logistics of running the project), and (5) scheduling (for example, setting up individual site calls). Using this node structure, two program coordinators coded the site diaries and swapped their coding for cross-checking. Discrepancies were discussed to achieve consensus.
The frequencies of different (sub-)categories of interactions between each site and the coordinating team during different phases of the participatory ergonomics process (pre-intervention, post-intervention, and total) were calculated. Poisson regression models were used to estimate the relative mean frequency of each (sub-)category, comparing post- to pre-intervention, as well as the relative mean frequency of each (sub-)category across sites, separately for each phase. Robust variance estimates were used to account for over- or under-dispersion in the data and clustering within site when comparing the different phases.
Semi-annual Surveys.
After each survey, each of the six domains of the dissemination process was scored (ranging from 1 to 5) by averaging the responses to all questions related to the domain. Next, a radar chart was created for each site to depict the changes of all six domain scores across the four surveys. The domain scores were then rescaled to a range of 1 (most negative and negative response), 2 (neutral response) and 3 (positive and most positive response); a hierarchical agglomerative cluster analysis with average linkage and Euclidean distance was conducted to define clusters of sites based on their responses to the pre- and post-intervention semi-annual surveys.
Impact of the Dissemination Process on Site Performance.
We correlated the site-specific change in blood culture rates, post- vs. pre-intervention, with dissemination process measures, including the number of interactions with the coordinating team, responses to the pre-intervention and post-intervention semi-annual surveys, and whether sites developed only CDS tools vs. making additional changes to other work system elements. For each process measure, a Poisson regression model was fit for the monthly blood culture rate as a function of a main effect of phase, post- vs. pre-intervention, a main effect of the process measure, and the interaction. Robust variance estimates were obtained to account for the clustering of monthly blood culture data within sites and for possible over-dispersion in the data.
RESULTS
Dissemination Process
Interactions Between Participating Hospitals and the Coordinating Team.
The total number of interactions between a site and the coordinating team ranged from 120 to 205. Table 3 shows the frequencies of different (sub-)categories of interactions during the pre- and post-intervention phases. During the post-intervention phase, the sites had 50% fewer emails and phone calls focusing on implementation facilitation (relative mean frequency = 0.5, p = 0.002), but more emails and phone calls focusing on data dialogue (1.3, p < 0.001), collection of site QI activities (2.0, p < 0.001), and scheduling (2.4, p = 0.001); along with more conference calls in total (1.5, p = 0.002), and more individual site calls (1.8, p < 0.001) with the coordinating team compared to the pre-intervention phase.
Table 3.
Frequencies of Interactions Between Sites and Coordinating Team
| Interactions | Pre-intervention Median (IQR) | Post-intervention Median (IQR) | Relative mean frequency† (95% CI) | p value |
|---|---|---|---|---|
| Email / phone calls | 47.5 (39, 53) | 63.5 (51, 77) | 1.3 (0.9, 1.9) | 0.143 |
| Data dialogue | 7 (4, 10) | 25 (17, 34) | 3.3 (2.0, 5.5) | < 0.001** |
| QI log collection | 3.5 (3, 4) | 6.5 (5, 8) | 2.0 (1.5, 2.5) | < 0.001** |
| Implementation facilitation | 14 (12, 16) | 5.5 (4, 10) | 0.5 (0.3, 0.8) | 0.002* |
| Project administration | 15.5 (12, 20) | 7.5 (6, 19) | 0.6 (0.4, 1.0) | 0.071 |
| Scheduling | 4.5 (3, 10) | 15 (14, 19) | 2.4 (1.4, 4.0) | 0.001** |
| Conference calls | 13.5 (11, 16) | 20 (19, 21) | 1.5 (1.1, 1.8) | 0.002* |
| All-site call | 6 (5, 8) | 7 (6, 8) | 1.1 (0.8, 1.5) | 0.512 |
| Individual site call | 7 (6, 8) | 13 (12, 14) | 1.8 (1.4, 2.3) | < 0.001** |
| Total | 60 (53, 71) | 84 (68, 97) | 1.3 (1.0, 1.9) | 0.078 |
p < 0.01;
p < 0.001.
Relative mean frequency of interactions, with 95% confidence interval (CI), derived from a Poisson regression model with main term for phase, post- vs. pre-intervention.
QI, quality improvement; IQR, interquartile ratio.
In addition, across sites, there were significant variations in the total number of emails and phone calls (median = 113, interquartile range = [103, 140], p < 0.001) and the numbers of emails and phone calls focusing on data dialogue (33, [24, 39], p = 0.003), implementation facilitation (22, [17, 26], p = 0.002), project administration (23, [21, 40], p < 0.001), and scheduling (22, [18, 28], p = 0.004).
Site Evaluation of the Dissemination Process.
The radar charts and the cluster assignments for the 14 sites based on the pre-intervention and post-intervention semi-annual survey responses are shown in Appendixes B and C, respectively. At the pre-intervention survey, the vast majority of sites responded positively to the leadership support, engagement of frontline clinicians, engagement of champions, and larger collaborative domains. Variation was observed across sites in responses to the resources and ongoing QI domains, with five, four, and five sites responding negative, neutral, and positive, respectively, for both domains. When clustering sites according to the post-intervention survey responses, the vast majority of sites had stable positive responses to the leadership support, engagement of champions, larger collaborative, and ongoing-QI domains. For the resources domain, three clusters were identified as stable neutral (n = 7), worsening positive (n = 5), or stable positive (n = 2). For the engagement of frontline clinicians domain, four clusters were identified as stable positive (n = 7), improving neutral (n = 4), worsening positive (n = 1), and increasing then decreasing neutral (n = 1) sites.
Dissemination Outcomes
Interventions and Implementation Strategies.
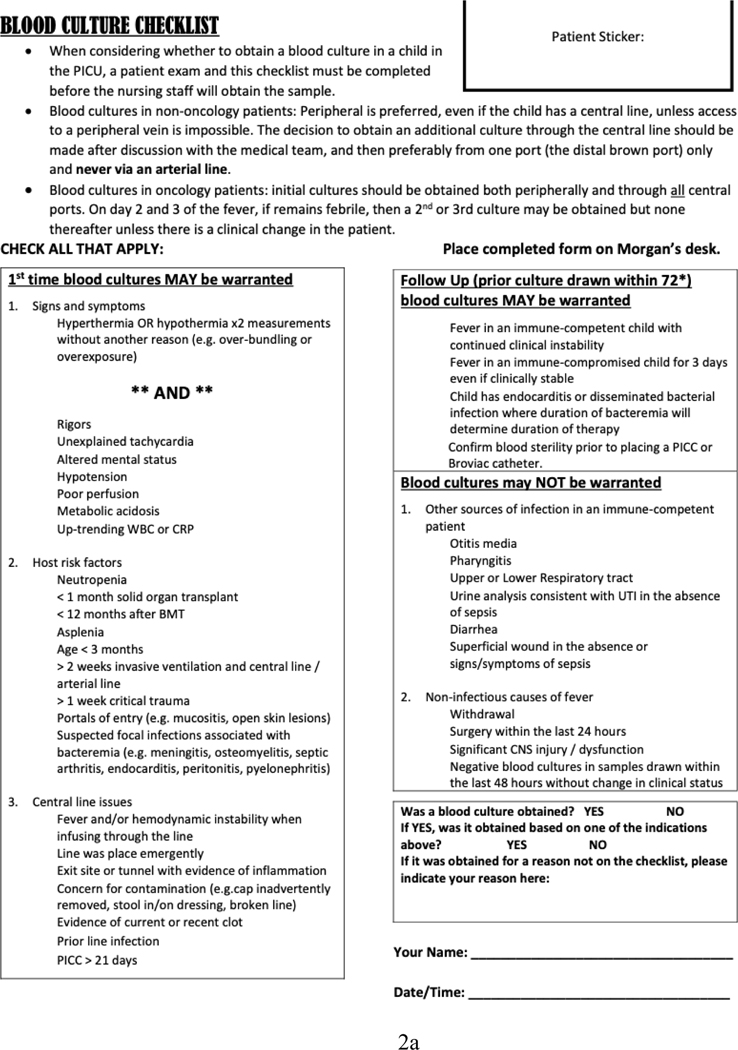
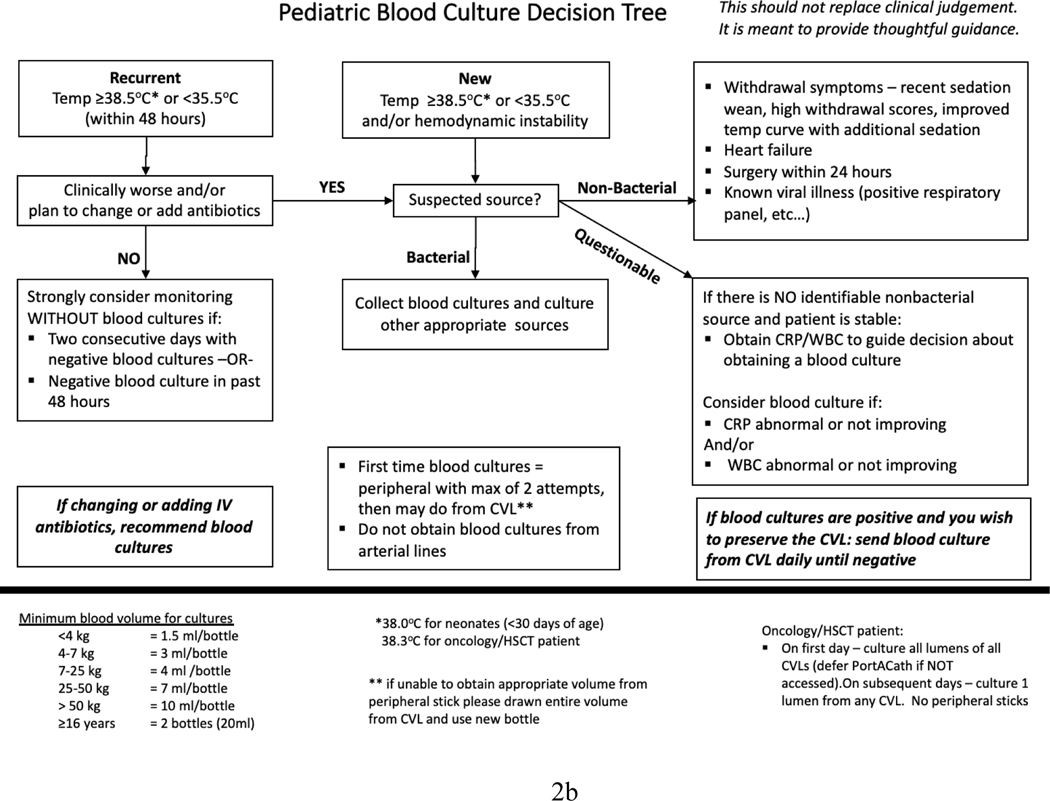
The 14 sites developed different interventions for improving blood culture ordering practices (Table 4). All sites included CDS tools as part of their interventions: four sites developed a checklist to screen for signs of sepsis and potential reasons for fever, assess risk factors, and determine if a blood culture was indicated (see Figure 2a for an example); five sites developed a flowchart to illustrate their blood culture decision algorithm (see Figure 2b for an example); and five sites developed both a checklist and a flowchart. While most sites placed paper-based CDS tools in the clinician workroom and/or at patient bedside, four sites (sites 3, 8, 9, and 11) integrated their CDS tools into the electronic blood culture order set and/or electronic health records. In addition to the CDS tools, eight sites made additional changes to their local work systems and processes, including revising institutional policies to standardize blood culture ordering practices, integrating discussions about blood cultures into daily rounds or huddles, introducing a peripheral blood culture kit, developing educational materials for families, and requiring patient exam before ordering a blood culture.
Table 4.
Interventions and Implementation Strategies
| Sites |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Intervention | |||||||||||||||
|
| |||||||||||||||
| Clinical decision support tool | Checklist | X | X | X | X | X | X | X | X | X | |||||
| Flowchart | X | X | X | X | X | X | X | X | X | X | |||||
| Other work system and process changes | X | X | X | X | X | X | X | X | |||||||
|
| |||||||||||||||
| Implementation strategies | |||||||||||||||
|
| |||||||||||||||
| Leadership and stakeholder engagement | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Clinician training | X | X | X | X | X | X | X | X | X | X | X | ||||
| Audit and feedback | X | X | X | X | X | X | X | X | X | X | X | ||||
| Stakeholder feedback | X | X | X | X | X | X | X | X | X | ||||||
| Reminder | X | X | X | X | X | X | X | X | X | X | X | X | |||
Figure 2.
Shown here are examples of a blood culture checklist (a) and a decision algorithm (b).
The 14 sites applied various strategies to facilitate the implementation of their interventions (Table 4): all sites engaged hospital leadership and key stakeholders through division meetings, meetings with specialist groups, and/or direct emails; 11 sites provided training on the use of the CDS tools, the updated blood culture policies, and/or the practices for obtaining peripheral blood cultures to clinicians; 11 sites shared data on compliance measures and blood culture use and discussed specific cases with clinicians; nine sites collected feedback from key stakeholders to continuously improve intervention design and implementation; and 12 sites sent periodic reminders through emails, newsletters, and/or unit posters to keep key stakeholders engaged and informed of the interventions.
Impact of the Dissemination Process on Site Performance.
Overall, BrighT STAR collaborative sites reduced blood culture use (monthly number of blood cultures per 1000 patient days) by 32.7% when comparing the post- to pre-intervention phases (p < 0.001), with an estimated 149.4 blood cultures per 1000 patient-days/month pre-intervention and an estimated 100.5 blood cultures per 1000 patient-days/month post-intervention.12
There was a weak negative correlation between the total number of interactions during the pre-intervention phase and the change in blood culture rate (Appendix D). For every five additional interactions, the reduction in blood culture rates decreased by ~1% (p = 0.057). There was no association between change in blood culture rate and pre-intervention site assessment of the six domains of the dissemination process (Appendix C). When comparing the eight sites that made additional changes to local work systems and processes to the six sites that only developed CDS tools, we found no statistically significant difference in the reduction of blood culture use (34% vs. 31%, p = 0.650).
DISCUSSION
In this study, we describe the dissemination of a QI program for optimizing PICU blood culture use to a large collaborative with 14 hospitals across the country. Guided by a PE conceptual framework and following a general dissemination process, participating hospitals were able to successfully implement the program and significantly reduce their blood culture use.12 Variations in the dissemination process, as well as in local interventions and implementation strategies, however, were observed across sites.
Different sites interacted with the coordinating team with different frequencies. The total number of interactions between a site and the coordinating team varied widely. A conceivable assumption was that sites having more interactions with the coordinating team were more engaged in the collaborative and achieved more reduction in blood culture use. However, our data showed weak negative correlations between the number of pre-intervention interactions and site performance—the more interactions a site had with the coordinating team during the pre-intervention phase, the smaller the reduction in blood culture use it achieved. A potential explanation might be that sites having smaller reductions in blood culture use had less QI experience or faced more challenges to implementing the program and, therefore, required more interactions with the coordinating team. Additional studies are needed to understand the specific underlying reasons and explore ways to meaningfully engage sites in QI dissemination.
In addition to the frequencies of interactions with the coordinating team, sites had different experiences with the six domains of the dissemination process. While most sites were able to secure leadership support, engage unit champions, and collaborate with other sites in the larger collaborative, some sites had challenges with consistently devoting sufficient resources, engaging frontline clinicians, and overcoming the impact of other QI projects throughout the dissemination process. The six domains have been identified and included in different implementation frameworks as key factors influencing QI programs.30–32 However, we did not find significant correlation between site experiences with the six domains and their performance in blood culture reduction. Future research is needed to provide more empirical evidence demonstrating the importance of these domains to the successful implementation of QI programs.
The PE conceptual framework used to guide the dissemination of the program emphasized the balance between program fidelity and adaptation.33 Following the general dissemination process and the evidence base supporting the reduction of blood culture use, participating sites were encouraged to engage local stakeholders to develop and implement their own interventions. As a result, sites developed CDS tools with varying content and format and made additional changes to their work systems and processes. It is unsurprising that no significant difference in blood culture reduction was identified between sites with different interventions and implementation strategies (for example, sites only developing CDS tools vs. sites making additional changes to the work systems and processes), since sites were expected to choose the interventions and implementation strategies that fit best with their local needs and challenges. The PE approach can be adapted and applied to facilitate the dissemination of other QI programs.
While the program was successfully disseminated to the large collaborative, we encountered some challenges during the dissemination process, including changes of local QI team members at times, difficulties in scheduling individual site calls and all-site calls with local QI teams, and difficulties in keeping all participating hospitals actively and consistently involved in the collaborative. The coordinating team mitigated these challenges by keeping a good relationship with local QI teams, scheduling and preparing meetings far enough ahead of time, adapting to the needs of participating hospitals, and appealing to higher authority if necessary. The dissemination process took place between January 2018 and March 2020 and, therefore, was not influenced by the COVID-19 pandemic. However, given the detrimental impact of the pandemic on health care systems (for example, capacity, patient population), further investigation is needed to track how the program is sustained at participating hospitals during and beyond the pandemic.
CONCLUSION
QI dissemination is important but challenging. In this study, we illustrated the application of a PE approach to the dissemination of a QI program for optimizing PICU blood culture use to a multi-site collaborative. Similar diagnostic stewardship programs, which promote judicious testing practices to inform safe, effective, and efficient patient management and treatment decisions, can be applied for a variety of diseases (for example, infectious diseases, respiratory diseases, cancer)34–36 across different settings, including low-income and middle-income countries.37
Different from a highly controlled clinical trial, sites participating in this collaborative were able to achieve the goal of reducing blood culture use by engaging local stakeholders to tailor their interventions and implementation processes. While sites had different experiences with the dissemination process (for example, leadership support, resources available, engagement of champions, engagement of frontline staff, impact of the larger collaborative, impact of ongoing QI projects), the number of pre-intervention interactions between a site and the coordinating team was the only process measure associated with site performance in blood culture reduction. The analysis, however, was limited by the small number of participating sites. Given the broad need for improving health care quality and safety, additional studies are needed to provide more empirical evidence on effective and feasible strategies for disseminating successful QI programs.
Supplementary Material
Acknowledgements
We would like to thank the QI team and PICU clinicians at each hospital for their participation in the BrighT STAR collaborative.
Funding
This project was supported by grant number R18HS025642 and R21HS025238 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr. Xie also receives support from the Centers for Disease Control and Prevention (R01CE003150). Dr. Woods-Hill also receives support from the National Heart, Lung, And Blood Institute of the National Institutes of Health (K23HL151381). Dr. Milstone also receives support from National Institute of Health (K24AI141580).
Footnotes
Declaration of Interest
None of the authors have a relevant conflict of interest.
References:
- 1.Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. New England Journal of Medicine 2004; 351(18):1838–1848. [DOI] [PubMed] [Google Scholar]
- 2.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. New England Journal of Medicine 2006; 355(26):2725–2732. [DOI] [PubMed] [Google Scholar]
- 3.White DB, Angus DC, Shields AM, et al. A Randomized Trial of a Family-Support Intervention in Intensive Care Units. N Engl J Med 2018; 378(25):2365–2375. [DOI] [PubMed] [Google Scholar]
- 4.Glasgow RE, Vinson C, Chambers D, et al. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health 2012; 102(7):1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhalgh T, Robert G, Macfarlane F, et al. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004; 82(4):581–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ 2008; 337:a1714. [DOI] [PubMed] [Google Scholar]
- 7.Rogers EM. Diffusion of Innovations. 5th ed. New York, NY: Free Press; 2003. [Google Scholar]
- 8.Kerner J, Rimer B, Emmons K. Introduction to the special section on dissemination: dissemination research and research dissemination: how can we close the gap? Health Psychol 2005; 24(5):443–446. [DOI] [PubMed] [Google Scholar]
- 9.Woods-Hill CZ, Fackler J, Nelson McMillan K, et al. Association of a Clinical Practice Guideline With Blood Culture Use in Critically Ill Children. JAMA pediatrics 2017; 171(2):157–164. [DOI] [PubMed] [Google Scholar]
- 10.Woods-Hill CZ, Lee L, Xie A, et al. Dissemination of a Novel Framework to Improve Blood Culture Use in Pediatric Critical Care. Pediatr Qual Saf 2018; 3(5):e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson JR, Haines H, Morris W. Participatory ergonomics. In: Wilson JR, Corlett EN, editors. Evaluation of Human Work. 3rd ed. Boca Raton, FL: Taylor & Francis; 2005. p. 933–962. [Google Scholar]
- 12.Woods-Hill CZ, Colantuoni EA, Koontz DW, et al. Association of Diagnostic Stewardship for Blood Cultures in Critically Ill Children With Culture Rates, Antibiotic Use, and Patient Outcomes: Results of the Bright STAR Collaborative. JAMA pediatrics 2022; 176(7):690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191(10):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiragu AW, Zier J, Cornfield DN. Utility of blood cultures in postoperative pediatric intensive care unit patients. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2009; 10(3):364–368. [DOI] [PubMed] [Google Scholar]
- 15.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect 2011; 77(3):233–236. [DOI] [PubMed] [Google Scholar]
- 16.Skoglund E, Dempsey CJ, Chen H, et al. Estimated Clinical and Economic Impact through Use of a Novel Blood Collection Device To Reduce Blood Culture Contamination in the Emergency Department: a Cost-Benefit Analysis. J Clin Microbiol 2019; 57(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haines H, Wilson JR, Vink P, et al. Validating a framework for participatory ergonomics (the PEF). Ergonomics 2002; 45(4):309–327. [DOI] [PubMed] [Google Scholar]
- 18.Cantley LF, Taiwo OA, Galusha D, et al. Effect of systematic ergonomic hazard identification and control implementation on musculoskeletal disorder and injury risk. Scand J Work Environ Health 2014; 40(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Guimaraes LBM, Anzanello MJ, Ribeiro JLD, et al. Participatory ergonomics intervention for improving human and production outcomes of a Brazilian furniture company. International Journal of Industrial Ergonomics 2015; 49:97–107. [Google Scholar]
- 20.Dale AM, Jaegers L, Welch L, et al. Evaluation of a participatory ergonomics intervention in small commercial construction firms. Am J Ind Med 2016; 59(6):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaegers L, Dale AM, Weaver N, et al. Development of a program logic model and evaluation plan for a participatory ergonomics intervention in construction. Am J Ind Med 2014; 57(3):351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess-Limerick B, Straker L, Pollock C, et al. Participative ergonomics for manual tasks in coal mining. International Journal of Industrial Ergonomics 2007; 37(2):145–155. [Google Scholar]
- 23.Xie A, Carayon P, Cox ED, et al. Application of participatory ergonomics to the redesign of the family-centred rounds process. Ergonomics 2015; 58(10):1726–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Eerd D, Cole D, Irvin E, et al. Process and implementation of participatory ergonomic interventions: a systematic review. Ergonomics 2010; 53(10):1153–1166. [DOI] [PubMed] [Google Scholar]
- 25.Xie A, Carayon P. A systematic review of human factors and ergonomics (HFE)-based healthcare system redesign for quality of care and patient safety. Ergonomics 2015; 58(1):33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pronovost PJ, Hudson DW. Improving healthcare quality through organisational peer-to-peer assessment: lessons from the nuclear power industry. BMJ Qual Saf 2012; 21(10):872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon-Woods M, Bosk CL, Aveling EL, et al. Explaining Michigan: developing an ex post theory of a quality improvement program. Milbank Q 2011; 89(2):167–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creswell J. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks, CA: Sage Publications; 2009. [Google Scholar]
- 29.Graneheim UH, Lundman B. Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse Education Today 2004; 24(2):105–112. [DOI] [PubMed] [Google Scholar]
- 30.Atkins L, Francis J, Islam R, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci 2017; 12(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glasgow RE, Estabrooks PE. Pragmatic Applications of RE-AIM for Health Care Initiatives in Community and Clinical Settings. Prev Chronic Dis 2018; 15:E02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keith RE, Crosson JC, O’Malley AS, et al. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: a rapid-cycle evaluation approach to improving implementation. Implement Sci 2017; 12(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen JD, Schelton RC, Emmons KM, et al. Fidelity and its relationship to implementation effectiveness, adaptation, and dissemination. In: Brownson R, Colditz G, Proctor E, editors. Dissemination and Implementation Research in Health: Translating Science to Practice. 2nd ed. New York, NY: Oxford University Press; 2018. p. 267–284. [Google Scholar]
- 34.Sick-Samuels AC, Woods-Hill C. Diagnostic Stewardship in the Pediatric Intensive Care Unit. Infect Dis Clin North Am 2022; 36(1):203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobler CC, Glasziou PP. Overdiagnosis in respiratory medicine. Respirology 2019; 24(10):939–941. [DOI] [PubMed] [Google Scholar]
- 36.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014; 65(6):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albarqouni L, Arab-Zozani M, Abukmail E, et al. Overdiagnosis and overuse of diagnostic and screening tests in low-income and middle-income countries: a scoping review. BMJ Glob Health 2022; 7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
Table 2 References
- 1.Xie A, Koontz DW, Voskertchian A, et al. Survey-based Work System Assessment to Facilitate Large-scale Dissemination of Healthcare Quality Improvement Programs. Pediatr Qual Saf 2020; 5(2):e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holden RJ, Carayon P, Gurses AP, et al. SEIPS 2.0: a human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics 2013; 56(11):1669–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.